Oral Thin Film - CD Formulation
Call Us:
- Home
- Services
- Oral Thin Film Formulation Development
- Quick Release Oral Thin Film Development
- Sublingual Thin Film Development
- Non-Disintegrating Buccal Film Development
- Fast-Disintegrating Buccal Film Development
- Mucoadhesive Sustained-Release Film Development
- 3D Printing Service of Oral Thin Film
- Single-Layer Oral Thin Film Development
- Double-Layer Oral Thin Film Development
- Multi-Layer Oral Thin Film Development
- One / Several APIs Oral Thin Film Development
- Novel Solvent-free Oral Thin Film Development
- Oral Thin Film Process Development
- APIs Screening and Characterization of Oral Thin Films
- Excipient Ingredient Evaluation of Oral Thin Film
- Preparation Method Screening of Oral Thin Films
- Solvent Casting Method for Oral Thin Film Preparation
- Hot Melt Extrusion Method for Oral Thin Film Preparation
- Semi-solid Casting Method for Oral Thin Film Preparation
- Solid Dispersion Extrusion Method for Oral Thin Film Preparation
- Rolling Method for Oral Thin Film Preparation
- 3D Printing Method for Oral Thin Film Preparation
- Oral Thin Film Characterization
- Oral Thin Film Organoleptic Evaluation
- Oral Thin Film Surface pH Testing
- Oral Thin Film Mechanical Properties Characterization
- Oral Thin Film Contact Angle Measurement
- Oral Thin Film Moisture Absorption Testing
- Oral Thin Film Swelling Properties Testing
- Oral Thin Film Transparency Testing
- Oral Thin Film Morphology Testing
- Oral Thin Film Content Testing
- Oral Thin Film Solid State Analysis
- Oral Thin Film Content Uniformity Testing
- Oral Thin Film Moisture Content Testing
- Oral Thin Film In Vitro Disintegration Testing
- Oral Thin Film In Vitro Dissolution Testing
- Oral Thin Film Adhesion Testing
- Oral Thin Film Irritation Study
- Oral Thin Film Release Kinetics Testing
- Oral Thin Film Efficacy Evaluation
- Oral Thin Film Analysis and Testing Services
- Oral Thin Film Manufacturing and Packaging Services
- Oral Thin Film Formulation Development
- Technologies
- Oral Thin Film Quality & Analytical Technologies
- Solubilization Technologies for Poorly Soluble Drugs
- Oral Thin Film Manufacturing Technologies
- Solvent Casting Technology for Oral Thin Film Manufacturing
- Hot Melt Extrusion Technology for Oral Thin Film Manufacturing
- Semi-solid Casting Technology for Oral Thin Film Manufacturing
- Solid Dispersion Extrusion Technology for Oral Thin Film Manufacturing
- Rolling Technology for Oral Thin Film Manufacturing
- 3D Printing Technology for Oral Thin Film Manufacturing
- Oral Thin Film Biologics Delivery Technologies
- Applications
- Oral Thin Film for Medical Areas
- Oral Thin Film for Cardiovascular Therapeutic Research
- Oral Thin Film for Pain Relief Research
- Oral Thin Film for Mood or Mental Disorder Therapy Research
- Oral Thin Film for Respiratory Disease Therapy Research
- Antihistamines Oral Thin Film Formulation Research
- Oral Thin Film for Erectile Dysfunction Therapy Research
- Oral Thin Film for Health Products
- Oral Thin Film for Food Industry
- Oral Thin Film for Cosmetic Industry
- Oral Thin Film for Medical Areas
- Order Online
- Company
- Inquiry

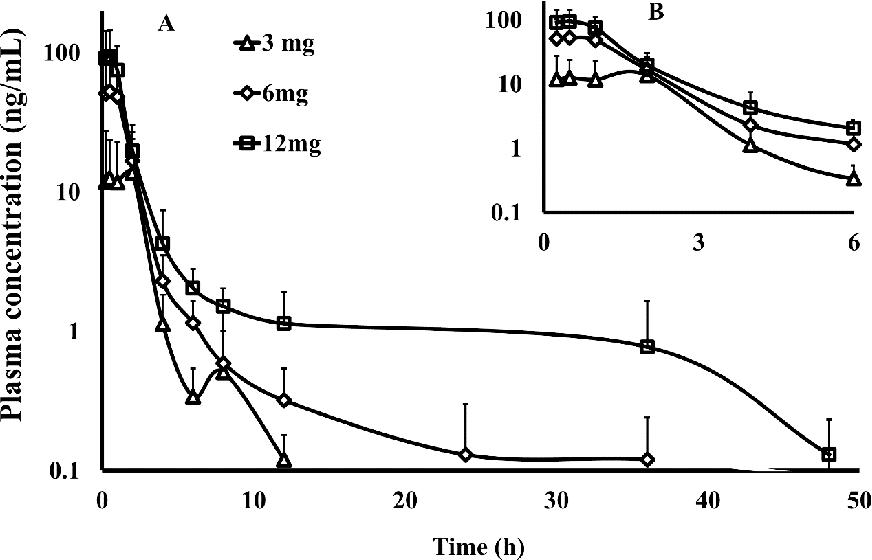 Fig.1 The plasma concentration–time curve wit in 48 h(A) and 6 h(B). (Zhiyuan Zhang, et al., 2018)
Fig.1 The plasma concentration–time curve wit in 48 h(A) and 6 h(B). (Zhiyuan Zhang, et al., 2018)
