Oral Thin Film In Vitro Dissolution Testing
Inquiry
In vitro dissolution testing is one of the most commonly used tests in drug quality control, as it enables the evaluation of the drug release profile and estimation of dosage form performance. However, for oral thin film preparations, there is no standardized pharmacopeia dissolution method or specification worldwide, and the pharmacopeia dissolution method for oral preparations is generally used. CD Formulation provides various oral thin film in vitro dissolution testing methods to meet the different testing requirements of customers, thus promoting the development of oral thin film products.
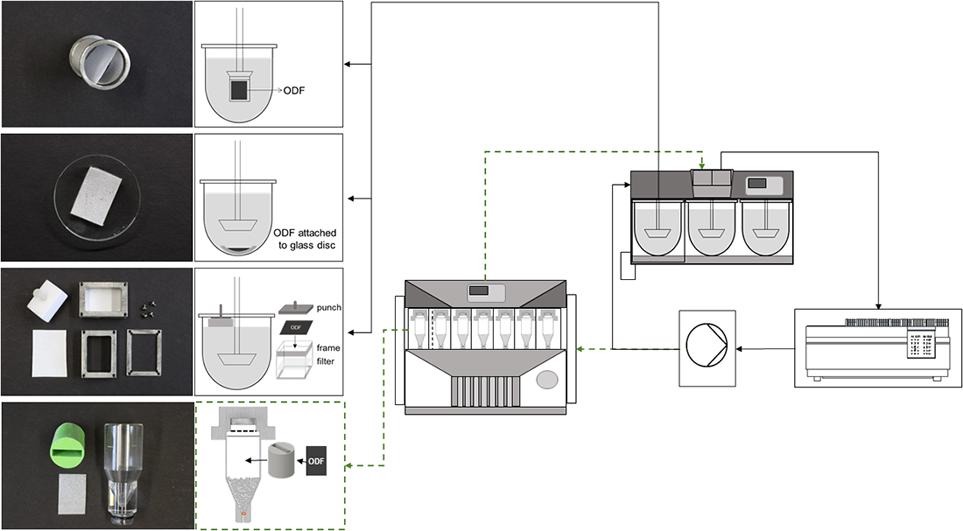 Fig.1 The dissolution testing of oral thin film. (Isabell Speer, et al., 2019)
Fig.1 The dissolution testing of oral thin film. (Isabell Speer, et al., 2019)
The Importance of Oral Thin Film In Vitro Dissolution Testing
In vitro dissolution testing is a critical test that measures the rate and extent of drug release from an oral thin film dosage form, which can provide essential information for quality control, formulation development, bioequivalence assessment, and predicting in vivo performance.
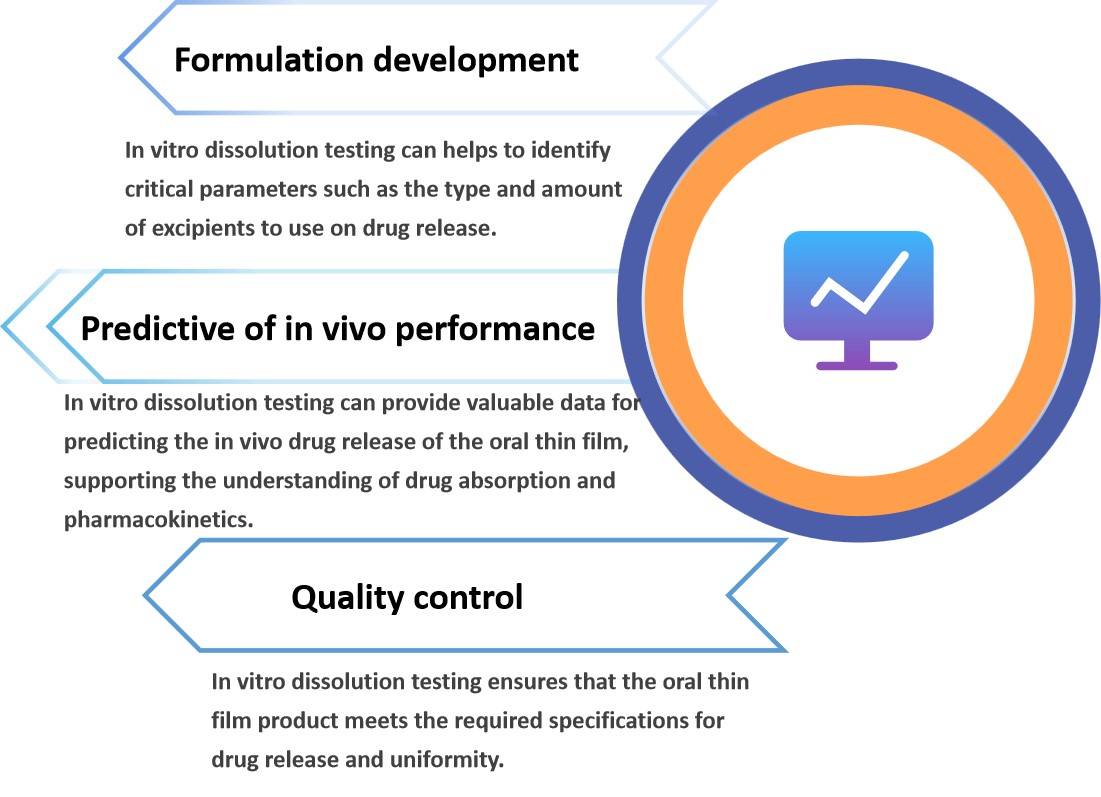 Fig.2 The Importance of Oral Thin Film In Vitro Dissolution Testing. (CD Formulation)
Fig.2 The Importance of Oral Thin Film In Vitro Dissolution Testing. (CD Formulation)
Our Oral Thin Film In Vitro Dissolution Testing Methods
Dissolution is a characteristic in vitro test method that reflects the characteristics of oral thin film, and it is also a necessary test item in the quality control of oral thin film preparation, but there is no universally recognized method. Our oral thin film in vitro dissolution test methods include the basket method, the paddle and glass disc (PGD) method, etc.
| In Vitro Dissolution Testing Methods |
Detailed Information |
| Basket (USP 1) Method |
The basket method is one of the common techniques used for conducting in vitro dissolution testing of oral thin film. Dissolution tests are performed with the basket method USP 1 at rotational speeds of 50 and 100 rpm using a dissolution tester, the dissolution medium is usually 500 mL of bi-distilled water or simulated saliva solution, pH 6.7, and the temperature is 37 ± 1 °C. |
| Paddle (USP 2) Method |
The paddle method is the most commonly used method for in vitro dissolution of oral thin film. The oral thin film is placed on a disk and immersed in a dissolution medium that is stirred using a paddle. The dissolution of the thin film is monitored over time by sampling the medium and analyzing the drug content. |
| Glass Disc Method |
In this method, a glass disc serves as the substrate for the oral thin film. The dissolution behavior of the thin film is evaluated by monitoring the release of the API into a suitable dissolution medium. |
| Rotating Disk Method |
In this method, the oral thin film is placed on a rotating disk and immersed in a dissolution medium. The rotation of the disk causes the medium to flow over the thin film, simulating the movement of the oral thin film in the gastrointestinal tract. The dissolution of the film is monitored by analyzing the drug content in the medium. |
Our Workflow of Oral Thin Film In Vitro Dissolution Testing
We have a professional oral thin film in vitro dissolution testing team and standardized testing procedures, which can quickly respond to customers' testing needs. Our test process usually includes the following steps:
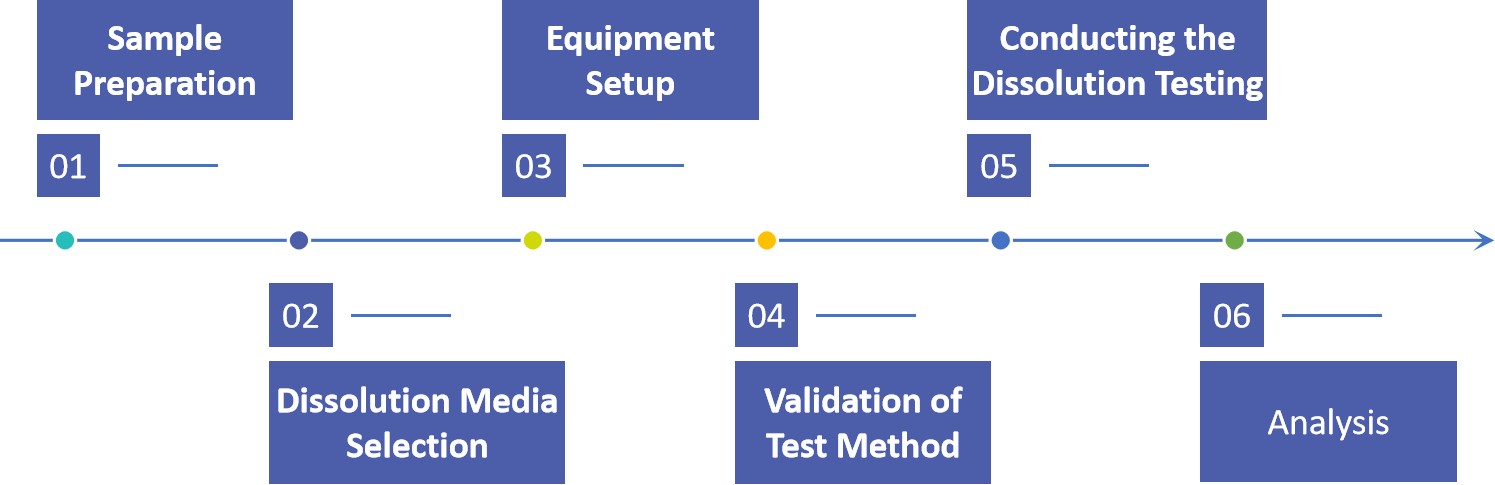 Fig.3 Process of Oral Thin Film In Vitro Dissolution Testing. (CD Formulation)
Fig.3 Process of Oral Thin Film In Vitro Dissolution Testing. (CD Formulation)
Sample Preparation: Accurately cut or section the films into uniform pieces to ensure consistency in the surface area and drug content for each sample tested.
Dissolution Media Selection: Select a suitable dissolving medium, such as simulated saliva, buffer solutions, or other media that mimic the composition and pH of saliva or buccal fluid.
Equipment Setup: Choose the appropriate dissolution testing apparatus based on the specific requirements of the oral thin film product.
Validation of Test Method: Establish parameters such as the apparatus type, rotation speed, sampling intervals, and analytical techniques for quantifying the drug release. Ensure the accuracy, precision, and reproducibility of the test method.
Conducting the Dissolution Testing: Place the prepared oral thin film samples in the dissolution apparatus and initiate the test according to the predetermined test conditions.
Analysis: Analyze the collected samples to measure the drug released from the oral thin film over time.
Advantages of Our Oral Thin Film In Vitro Dissolution Testing
- Specialized Expertise: Our team of experienced scientists and researchers with a deep understanding of in vitro dissolution testing methods and regulatory requirements.
- State-of-the-art Facilities: We are equipped with cutting-edge dissolution testing equipment and instrumentation, allowing for precise and accurate analysis of oral thin film dissolution behavior.
- Comprehensive Data Analysis: Our team conducts thorough data analysis and generates detailed dissolution profiles. This information aids in the assessment of thin film products' performance and potential for clinical efficacy
- Diversified Testing Methods: We not only have conventional in vitro disintegration testing equipment but also newly developed equipment for customers to choose from.
- Timely and Reliable Results: Our commitment to delivering reliable data within established timelines.
Published Data
Technology: In-vitro Dissolution Study
Journal: International Journal of Drug Delivery Technology
IF: 4.0
Published: 2021
Results: In this study, dissolution profiles of Meloxicam oral films were done for oral film formulated from Meloxicam nanoparticles (FMX1) and for oral film prepared from Meloxicam raw material using 500 mL simulated saliva solution PH 6.75 at 50 rpm and 37°C. The accumulative percentage of the release of Meloxicam from film containing FMX1 in three minutes was 55.5% and reached 100% at the time of 5 minutes. While accumulative percentage of the release of raw Meloxicam film in three minutes was 25.2% and did not reach 100% of release due to poor solubility of Meloxicam in simulated saliva, While oral film prepared with Meloxicam nanoparticles (FMX1) gave the best dissolution profile and quick release of Meloxicam nanoparticles.
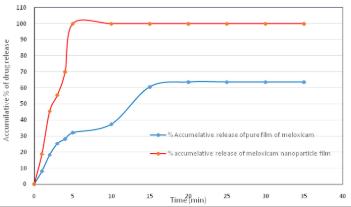 Fig.4 In vitro release profile of oral film in simulated saliva. (Malath H. Oudah, et al., 2021)
Fig.4 In vitro release profile of oral film in simulated saliva. (Malath H. Oudah, et al., 2021)
In vitro dissolution testing is a critical test that measures the rate and extent of drug release from an oral thin film. CD Formulation offers high-quality in vitro dissolution testing services for oral thin films. If you require our oral thin film in vitro dissolution testing services, please contact us by phone or email, and our colleagues will get back to you within three working days.
References
- Isabell Speer, Maren Preis, et al. Dissolution testing of oral film preparations: Experimental comparison of compendial and non-compendial methods. International Journal of Pharmaceutics. 2019, Vol (561):124-134.
- Malath H. Oudah, Fatimah M. H. Waiset, et al. Preparation and Evaluation of Meloxicam Nanoparticles as Oral Thin Film. International Journal of Drug Delivery Technology. 2021, Vol(11):676-684.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 The dissolution testing of oral thin film. (Isabell Speer, et al., 2019)
Fig.1 The dissolution testing of oral thin film. (Isabell Speer, et al., 2019) Fig.2 The Importance of Oral Thin Film In Vitro Dissolution Testing. (CD Formulation)
Fig.2 The Importance of Oral Thin Film In Vitro Dissolution Testing. (CD Formulation) Fig.3 Process of Oral Thin Film In Vitro Dissolution Testing. (CD Formulation)
Fig.3 Process of Oral Thin Film In Vitro Dissolution Testing. (CD Formulation) Fig.4 In vitro release profile of oral film in simulated saliva. (Malath H. Oudah, et al., 2021)
Fig.4 In vitro release profile of oral film in simulated saliva. (Malath H. Oudah, et al., 2021)
