One / Several APIs Oral Thin Film Development
Inquiry

As a new drug delivery system, oral thin film can provide rapid, local or systematic drug delivery, which is a very thin film composed of a mono or multi-layer polymeric matrix that is applied in the mouth. Usually, one / several APIs are dissolved or suspended in a polymer matrix to make an oral thin film containing one / several APIs. Most of the early oral thin films contain one API, with the progress of science and technology, we have been able to develop oral thin films containing several APIs, including water-soluble and insoluble. As an industry leader in new drug delivery system services, CD Formulation provides comprehensive development services for one / several APIs oral thin film development based on our advanced technology platform and development expertise.
Why Develop One / Several APIs Oral Thin Film?
- The one API oral thin film has the advantages of low production cost, simple production process, and stable property, which is usually the preferred dosage form.
- The volume of the oral thin film is small, which determines the drug load is not large, the APIs of the general drug account for 5% to 30% of the entire preparation, which limits the therapeutic effect of the drug, to comply with the patient's condition, it is necessary to combine 2 or several APIs to make oral thin films, improve the therapeutic effect.
- For the oral thin films containing several APIs, the film-forming material as a carrier, on the one hand, can well ensure the uniform distribution of drugs, and will not affect the effectiveness of the respective drugs. On the other hand, compared with oral liquid preparation and injection, the interaction between drugs after film formation is less, which can ensure the stability of individual API and the stability of drugs.
Our One / Several APIs Oral Thin Film Development Services
As a global leader in oral film development, CD Formulation has extensive experience in the development of one / several API oral films. Our team of experienced experts is dedicated to helping customers resolve any difficulties and challenges that may arise during the development of one/several APIs oral film formulations.
 Fig. 1 Process of One / Several APIs Oral Thin Film Development. (CD Formulation)
Fig. 1 Process of One / Several APIs Oral Thin Film Development. (CD Formulation)
Formulation Design
The development of oral thin films requires specific functional excipients, and in specific products, the types and proportions of excipients must be adjusted to achieve the desired characteristics. In the formulation design stage, we focus on the properties of the APIs. When there are more than two APIs, and there are incompatible ingredients or insoluble ingredients among these APIs, we will first choose 3D printing technology.
Production Technology
In mass production, we usually use a film coating machine to coat the film, which can better control the thickness and uniformity of the film, to obtain an accurate dose of oral thin film.
Quality Control
According to the characteristics of one / several APIs oral thin film and the existing domestic and foreign quality control conditions for the development and research of oral thin films, we carried out quality control from the aspects of apparent evaluation, mechanical performance evaluation and drug loading performance evaluation.
Manufacture
We have broken through several technical barriers in the process of oral thin film formulation and industrialization research and developed an oral film containing one / several APIs, which can provide the latest generation of solid dispersion technology to further stabilize the stability of APIs in an oral thin film so that it can maintain close to infinite stability. We established a leading oral thin films R&D and industrialization technology platform, that can provide customers with oral thin films product development and production, including small trials, and pilot tests to process scale-up production of the whole platform industry chain, to help you accelerate the product market.
Our Platforms for One / Several APIs Oral Thin Film Formulation Development
| Technologies & Platforms |
Specifics Contents |
| Formulation Design Platform |
The quality by design (QbD) approach was applied for optimizing the formulation of prepared oral thin films. The starting formulation was based on earlier experiments and contained the film forming agents and the plasticizers. To optimize this formulation a quality target product profile was established in which critical quality attributes (CQAs) such as mechanical properties and disintegration time were defined and quantified. Response surface methodology (RMS) was used to evaluate the effects of the CQAs of the final product. |
| Mechanical Properties Testing Platform |
The tensile test is the most widely used method for testing the mechanical characteristics of oral thin films. The other available test is the folding endurance test. We established an automatic method for determining the folding endurance number, and to compare the resulting FE numbers with the tensile properties. |
Our Advantages in One / Several APIs Oral Thin Film Development
- We are very experienced in increasing the drug load of APIs and making several insoluble APIs into oral thin films.
- Our team of experts can develop one / several APIs oral thin film based on the customer's requirements the properties of the APIs, and the patients to whom they are to be applied.
- Our team of experts is adept at using the Quality of Design (QbD) approach to optimize the formulation of one / several oral thin film using software.
- We have a team of highly skilled scientists and researchers with extensive experience and expertise in developing one / several APIs oral thin film drug delivery systems.
- We have an advanced, comprehensive one / several APIs oral thin film drug development platform to maximize experimental success.
Published Data
Technology: Development of orodispersible films containing different film forming polymers
Journal: International Journal of Pharmaceutics
IF: 5.8
Published: 2015
Results: In this research, two different BCS class II drugs were incorporated into orodispersible films in crystalline form. Furthermore, physical stability of films was examined at two different storage conditions.
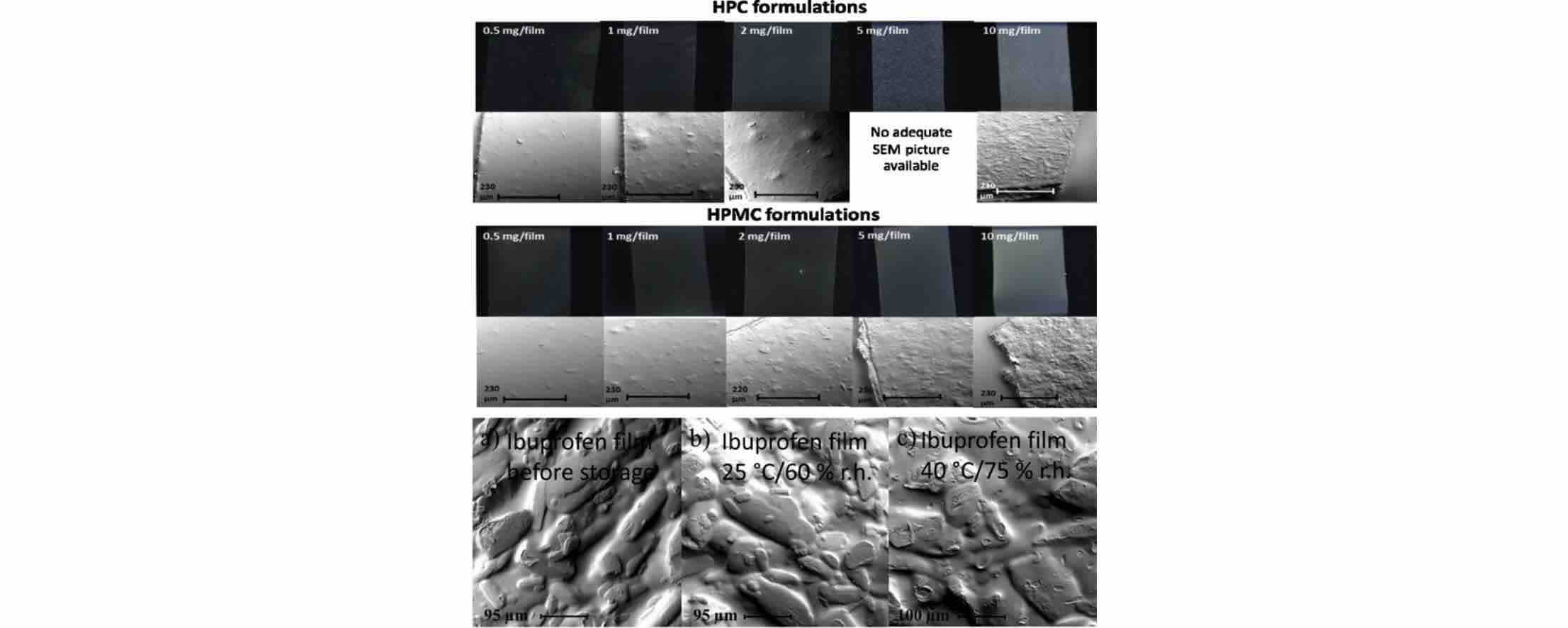 Fig.2 Development of orodispersible polymer films containing poorly water soluble active pharmaceutical ingredients. (Christina Woertz, et al., 2015)
Fig.2 Development of orodispersible polymer films containing poorly water soluble active pharmaceutical ingredients. (Christina Woertz, et al., 2015)
CD Formulation has rich experience in the R&D and industrialization of oral thin films and has mastered the international leading R&D system, experimental model, production process technology and proprietary technology to develop high-quality one / several APIs oral thin film for customers. If you are interested in our one / several APIs oral thin film drug delivery system development services, please don't hesitate to contact us for more information, our colleagues will reply to you within three working days.
References
- Zhuoqin Jiang, Changzhao Jiang, et al. Research progress of oral thin films and overview of commercially available drugs. Chinese Journal of New Drugs. 2020, Vol (29).
- Christina Woertz, Peter Kleinebudde. Development of orodispersible polymer films containing poorly water soluble active pharmaceutical ingredients with focus on different drug loadings and storage stability. International Journal of Pharmaceutics. 2015, Vol (493):134-145.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services





