Novel Solvent-free Oral Thin Film Development
Inquiry
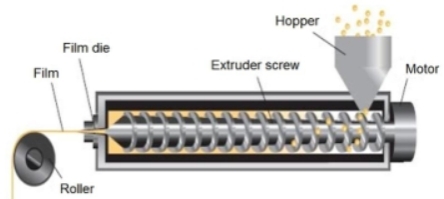
Oral thin films are a modern oral drug delivery method developed based on transdermal patch technology. Like tablets, the physical structure and low moisture content of the film make it an inhospitable environment for microbial growth, thus minimizing the need for preservatives and providing greater stability and shelf life. In the manufacture of oral thin films, solvent casting, and hot-melt extrusion are dominant. Hot-melt extrusion is a repeatable, continuous, which is often used to produce a novel solvent-free oral thin film. CD Formulation has a professional solvent-free mouth soluble film technology development platform, providing the whole process service from formula design, quality control to commercial production.
Why Develop Novel Solvent-free Oral Thin Film?
- The production process of the novel solvent-free oral thin film does not involve solvents such as ethanol, and the production process of this film is good repeatability, uniform content, fewer processing steps, more environmentally friendly, safer, and more cost-effective.
- The novel solvent-free oral thin film production process eliminates the use of water and other solvents, minimizing the impact of powder fluidity and compressibility.
- The novel solvent-free oral thin film achieves its special properties (increasing drug solubility, prolonging drug release, masking taste, etc.).
Our Novel Solvent-free Oral Thin Film Development Services
Formulation Design
In general, the preparation of oral thin film requires three main components, film-forming polymer, APIs, and plasticizer. To ensure the flexibility of the solvent-free oral thin film and easier to act on the application site, we will add other ingredients such as biological adhesives in the development process of the solvent-free oral thin film to ensure that the film can adhere to the action site within a certain period, allowing the drug to be absorbed or work.
Production Process
The production of solvent-free oral thin film is to heat the APIs and excipients after mixing, and then extrude the hot soluble substance through the mold hole to form a rod, and then press it into a film with a cooling roller. In this process, solvent-free heat treatment is used to realize the mixing and film formation of each component. Due to the high temperature involved in the production process, the thermal degradation of the drug or excipient used in the preparation may occur, so we perform a thermogravimetric analysis to evaluate the thermal behavior of the formula and select the appropriate processing temperature range.
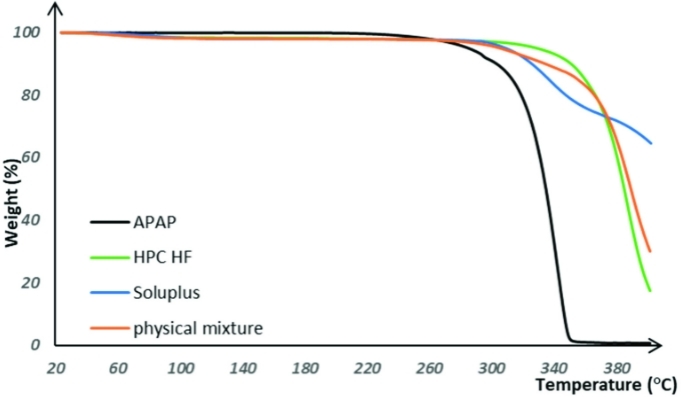 Fig.1 The thermal degradation curves for raw materials and physical mixtures. (Jiaxiang Zhang., et al., 2021)
Fig.1 The thermal degradation curves for raw materials and physical mixtures. (Jiaxiang Zhang., et al., 2021)
Quality Control
Our experienced technicians carry out comprehensive quality control, including appearance, film thickness, content and content uniformity, texture analysis, film disintegration, dissolution in simulated saliva, etc.
Manufacture
We have very rich experience in pilot transfer of solvent-free oral thin film, and we also have strong manufacturing capabilities.
Our Platforms for Novel Solvent-free Oral Thin Film Formulation Development
| Technologies & Platforms |
Specifics Contents |
| 3D Printed Technology Platform |
3D Printed Technology has emerged as an attractive technology to fabricate a wide array of pharmaceutical dosage forms, builds objects layer by layer from a computer-aided digital design. |
| Thermogravimetric (TGA) Analysis Platform |
Since a solvent-free thermal process is utilized in the current investigation to achieve molecular level mixing and film forming, a thermal degradation of the drug or excipients used in the formulations might happen due to the high processing temperature of the casting, extrusion, and printing. Thus, TGA is conducted to assess the thermal behavior of the formulations and select a suitable processing temperature range before performing actual HME and 3D printing. |
| Films Morphology Analysis Platform |
The films are cut into 10 × 10 mm pieces, and a digital caliper is used to determine the thickness and dimensions of the films. The weight of extruded and printed filaments is measured using an analytical balance. Furthermore, an optical microscope is used to image the samples. |
Our Advantages in Novel Solvent-free Oral Thin Film Development
- Our team of experts used this novel solvent-free oral thin film development technology that can effectively prevents bitter drugs from coming into contact with the patient's taste buds and improves patient compliance.
- We can skillfully combine HME with 3D printing technology to develop novel solvent-free oral thin film with better quality (such as faster dissolution of drugs).
- We can develop personalized solutions based on the characteristics and needs of customers' drugs.
- We provide end-to-end solutions for novel solvent-free oral film, from early project evaluation, formulation development to product launch.
Published Data
Technology: Development and Evaluation of Amorphous Oral Thin Films Using Solvent-Free Processes
Journal: Pharmaceutics
IF: 5.4
Published: 2021
Results: In this study, the main objective is to develop oral thin film (OTF) formulations using novel solvent-free approaches, including additive manufacturing (AM), hot-melt extrusion, and melt casting. This study suggested that HME combined with 3D printing can potentially improve the physical properties of formulations and produce OTFs with preferred qualities such as faster dissolution rate of drugs.
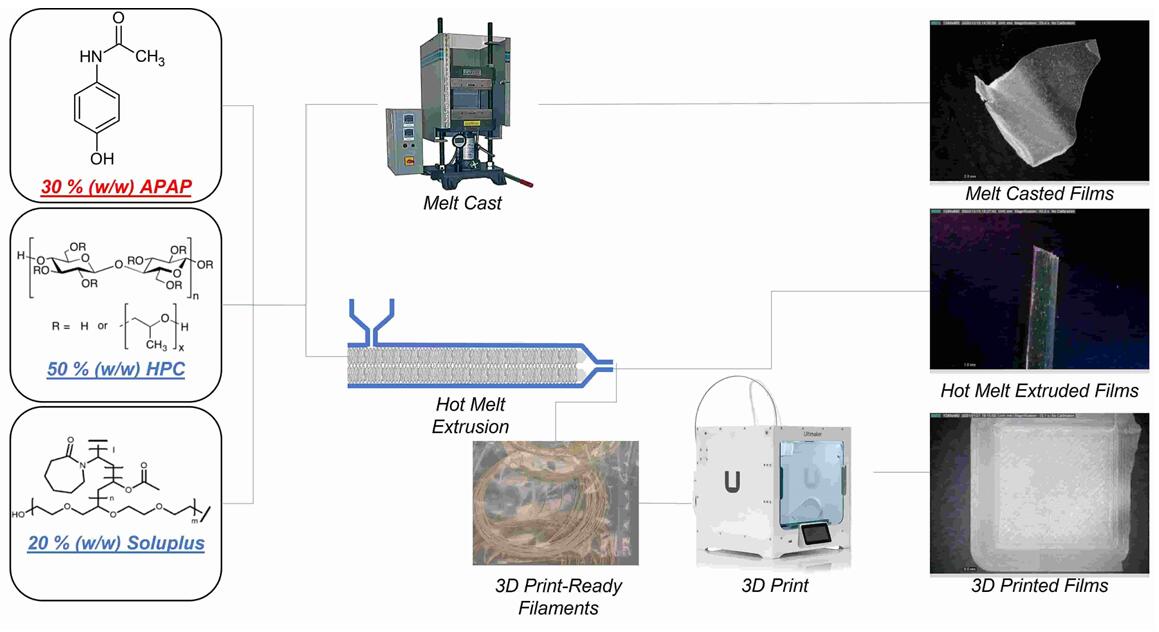 Fig.2 Development and Evaluation of Amorphous Oral Thin Films Using Solvent-Free. (Jiaxiang Zhang., et al., 2021)
Fig.2 Development and Evaluation of Amorphous Oral Thin Films Using Solvent-Free. (Jiaxiang Zhang., et al., 2021)
CD Formulation is at the forefront of innovative drug delivery system development, providing customized solutions for the development of novel drug delivery systems. Our expertise lies in the use of advanced technology and processes to design safe, efficient and reliable novel solvent-free oral thin film. If you have a requirement about our novel solvent-free oral thin film development services, please contact us by phone or email, and our colleagues will reply to you within three working days.
References
- Jiaxiang Zhang, Anqi Lu, et al. Development and Evaluation of Amorphous Oral Thin Films Using Solvent-Free Processes: Comparison between 3D Printing and Hot-Melt Extrusion Technologies. Pharmaceutics. 2021,13.
- Ahmed Abd El-Bary, Ibranhim Al Sharabi, et al. Effect of casting solvent, film forming agent and solubilizer on orodispersible films of a polymorphic poorly soluble drug. An in vitro/in silico study. Drug Dev.ind.Pharm. 2019,45(11):1751-1769.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services






