Non-Disintegrating Buccal Film Development
Inquiry
The non-disintegrating buccal film will not dissolve when it comes into contact with saliva, and the APIs are continuously released under control. It is developed as a promising dosage form, which has prominent advantages because of drug delivery through the buccal mucosa. CD Formulation has many years of experience in the development of oral thin films, and our professional technical teams and advanced development platform can efficiently develop the non-disintegrating buccal film.
 Fig.1 Rizatriptan loaded hydrogel-based mucoadhesive buccal film. (Shery Jacob, et al., 2021)
Fig.1 Rizatriptan loaded hydrogel-based mucoadhesive buccal film. (Shery Jacob, et al., 2021)
Why Develop Non-Disintegrating Buccal Film?
- The sublingual and buccal delivery of a drug via thin films has the potential to improve the onset of action, lower the dosing, and enhance the efficacy and safety profile of the medicament.
- Non-disintegrating buccal film is more advantageous as our technical teams can deliver sustained and stable release of drug ingredients through customized drug release profiles.
- Each strip guarantees the accuracy of the dose administered compared to drop or syrup preparations.
- Compared with tablets, the fundamental benefit of a non-disintegrating buccal film is that its wide surface area enables rapid moistening of the film and rapid and constant release of active ingredients, thereby accelerating drug absorption.
- The buccal mucosa has an abundant blood supply and is an ideal and rapid location for drug absorption.
Our Non-Disintegrating Buccal Film Development Services
Formulation Design
During the formulation design phase of the non-disintegrating film, we need to focus on the adhesion testing, our team of experts will evaluate the suitability of excipients and adhesion properties so that some new copolymers can be selected as biomimetics for adhesion tests more cost-effectively. In addition, we also pay attention to the compatibility of film formers, plasticizers, masking technologies, and APIs to achieve the characteristics of the desired non-disintegrating buccal film properties.
Preparation Method Screening
We focus on the performance testing and safety evaluation of buccal film. We select the appropriate casting technology based on the properties and characteristics of the non-disintegrating buccal film. At the same time, we also analyze many aspects to ensure the applicability of the method.
Quality Control
Our oral thin film technology platform is equipped with the world's top brands of equipment, including an X-ray powder diffractometer, Raman spectrometer, thermogravimetric differential thermal meter, gas adsorption detector, texture meter, rheometer, high-performance liquid chromatography, vacuum emulsion tank, electrostatic spinning machine, coating machine, and scutter, etc., in the entire development and production process to implement strict quality control.
Manufacture
In the whole process of pilot development, pilot scale, and commercial production of oral thin film preparations, we can realize all-round control of key process parameters of non-disintegrating buccal film.
Our Technologies for Non-Disintegrating Buccal Film Development
| Technologies |
Specifics Contents |
| Microencapsulate Technology |
This encapsulation technology is used in particular for products and ingredients for the food industry. Our technical teams microencapsulate drugs that are difficult to accept, such as bitter taste, into some pH-sensitive polymers to achieve good taste masking and improve patient compliance. |
| Solvent Evaporation Technology |
Solvent evaporation involves emulsification of polymer in aqueous phase and dispersion in a volatile solvent like dichloromethane, chloroform, and ethyl acetate. Then the solvent is evaporated using high temperature, vacuum, or by continuous stirring. |
| Solvent Extraction Technology |
Solvent extraction is a classical analytical technique used to determine the contents of various inorganic and organic species. Inorganic compounds are usually extracted after complexation with organic ligands. |
Our Advantages in Non-Disintegrating Buccal Film Development
- Customized Drug Release Profiles: Our non-disintegrating buccal film development services enable the customization of drug release profiles, allowing for precise modulation of therapeutic effects and ensuring consistent drug release, ensuring that the integrity of the drug remains unchanged over time.
- Excipient Screening Service: The non-disintegrating buccal film is flexible, it also shows the fragile, effervesced granule property, so our expert teams play special attention to the selection of plasticizers to ensure the flexibility of the buccal film and prevent brittleness.
- Professional Taste Masking Technology: Taste masking is a necessary condition for the commercial success of non-disintegrating buccal film. We have professional taste mask technical teams, our technical teams can microencapsulate drugs with unacceptable taste, such as bitterness into some pH-sensitive polymers through solvent evaporation and solvent extraction techniques. These polymer microspheres exhibit effective taste masking.
- Advanced Development Platform: We have an advanced and comprehensive non-disintegrating buccal film drug development platform to maximize experimental success.
Published Data
Technology: Solvent Casting technology
Journal: International Journal of Pharmaceutics
IF: 5.8
Published: 2021
Results: In this study, biomimetic materials were evaluated as a replacement for buccal mucosa in mucoadhesion testing and potential adhesives were compared regarding their suitability to increase the adhesion of hypromellose-based oromucosal films. Gelatin gels, as possible biomimetics, failed to mimic the buccal mucosa.
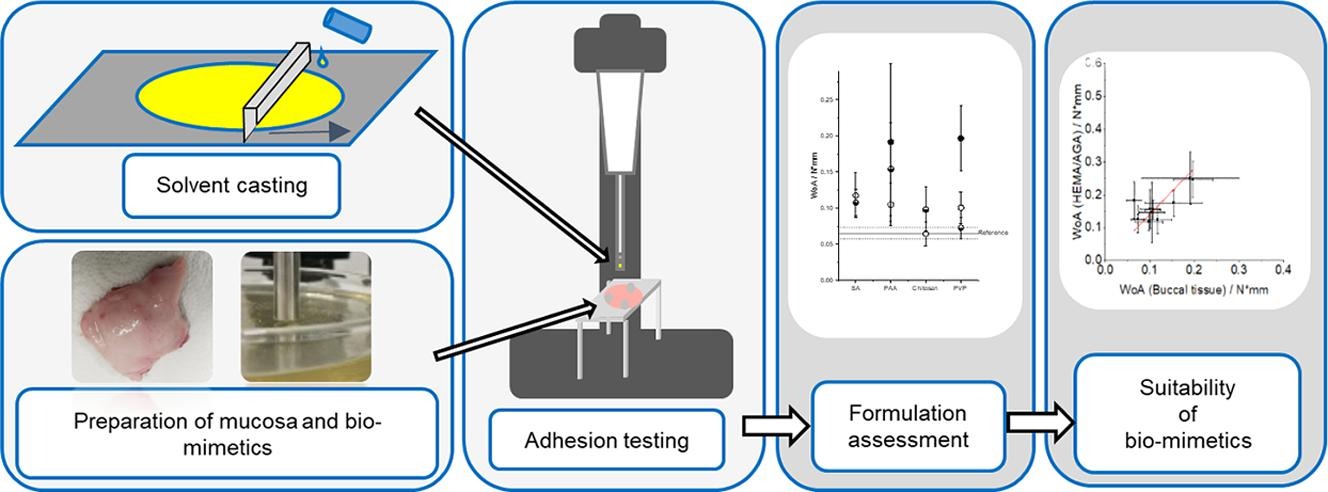 Fig.2 Development of buccal film formulations and their mucoadhesive performance in biomimetic models. (Anja Göbel, et al., 2021)
Fig.2 Development of buccal film formulations and their mucoadhesive performance in biomimetic models. (Anja Göbel, et al., 2021)
Relying on the advanced technology platform and professional research and development team, CD Formulation constantly overcomes difficulties and explores innovations to provide effective development solutions for non-disintegrating buccal film drug delivery systems. If you have any requirements about our non-disintegrating buccal film development services, please contact us by phone or email, our colleagues will reply to you within three working days.
References
- Shery Jacob, Anroop B. Nair, et al. AnUpdated Overview of the Emerging Role of Patch and Film-Based Buccal Delivery Systems. Pharmaceutics. 2021:02-39.
- S.M.Shahidulla, Ayesha Begum, et al. BUCCAL FILM: AN UPDATED OVERVIEW. INTERNATIONAL JOURNAL FOR INNOVATIVE RESEARCH IN MULTIDISCIPLINARY FIELD. 2022, Vol (8):132-140.
- Lewis Shipp, Fang Liu, et al. Buccal films: A review of therapeutic opportunities, formulations & relevant evaluation approaches. Journal of Controlled Release. 2022:1071–1092.
- Anja Göbel, Jessica Bassi da Silva, et al. Development of buccal film formulations and their mucoadhesive performance in biomimetic models. International Journal of Pharmaceutics. 2021, Vol (610).
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services





