Multi-Layer Oral Thin Film Development
Inquiry
Multi-layer oral thin film is made of a multilayer polymer matrix. They are typically water-based soluble polymers used for rapid drug delivery, providing immediate, regional, or systemic effects. These polymer-based films containing one or more active pharmaceutical ingredients (APIs), when applied to the mucosa, in particular the oral mucosa, the APIs can be delivered directly into the mucosa. The good blood supply of the oral mucosa ensures rapid transfer of the APIs into the bloodstream. Most of APIs are absorbed by the mucosa, thus avoiding the first-pass effect that occurs with conventional dosage forms. CD Formulation provides professional and reliable formulations of multi-layer oral thin film development services.
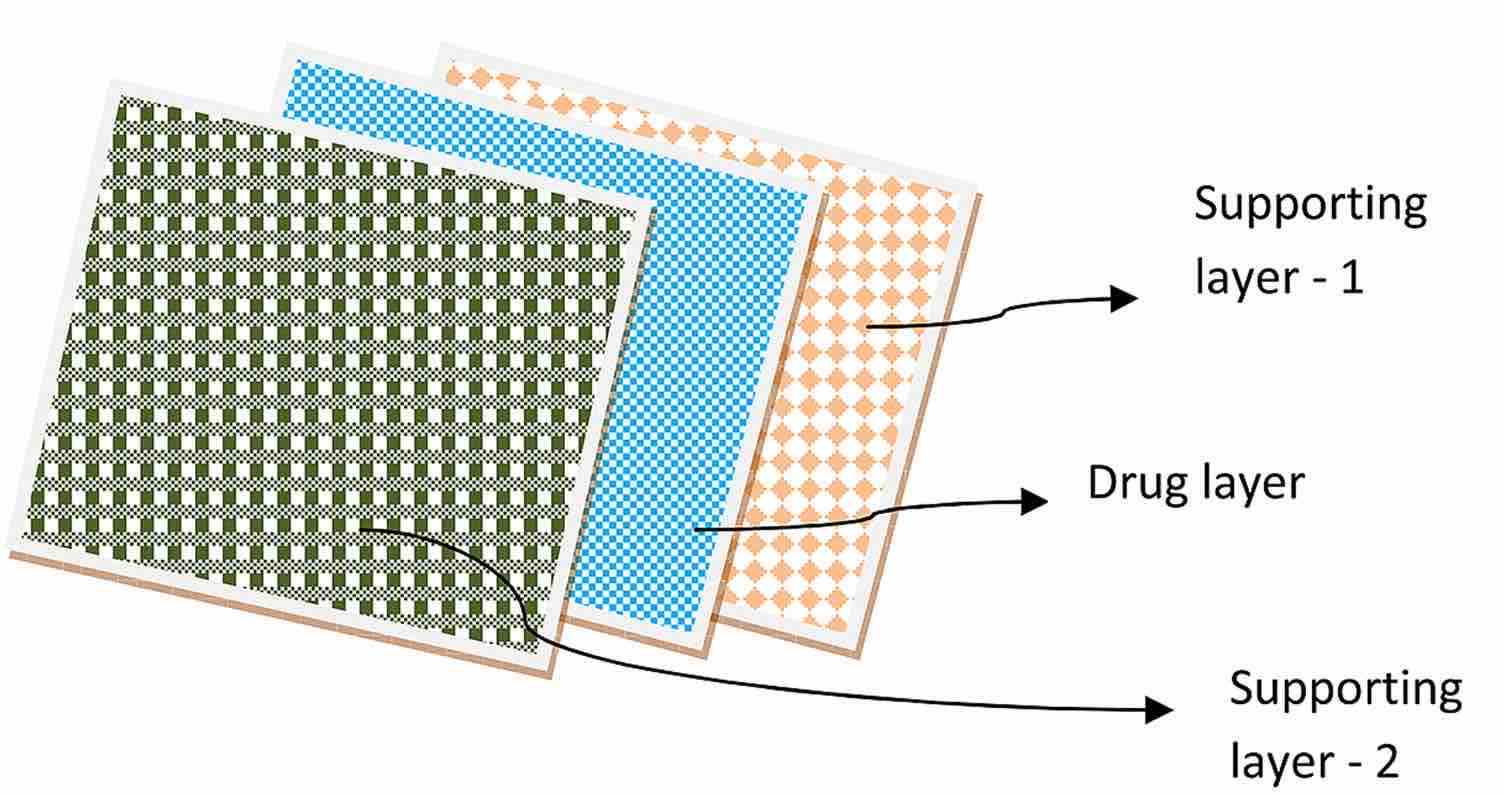 Fig.1 Schematic Diagram of Multilayered Oral Thin Films. (Maram Suresh Gupta., etc., 2020)
Fig.1 Schematic Diagram of Multilayered Oral Thin Films. (Maram Suresh Gupta., etc., 2020)
Why Develop Multi-Layer Oral Thin Film?
Multi-layer oral thin film, usually prepared using self-assembly technology, is an emerging field of drug delivery and a promising option to achieve desired goals, this drug delivery system has multiple advantages.
- It can be loaded with multiple APIs, incompatible APIs can be separated, and the layers can be formulated to have the same or different dissolution rates.
- It can form a combined system, multi-layer films will hinder the diffusion of drugs, thereby achieving sustained release of drugs.
- It is based on polyelectrolyte bonds between oppositely charged polymer molecules. Drug molecules are often incorporated during self-assembly processes, providing uniform loading in multi-layer structures.
- Since the taste of many APIs is objectionable to most people, multi-layer oral thin film drug delivery systems can add taste-masking agents to the matrix containing APIs to form a combination release of drugs, thereby achieving a good taste-masking effect.
- It can achieve high drug-loading capacity.
Our Multi-Layer Oral Thin Film Development Services
Formulation Design
The formulation design phase is critical to determine the basic structure of the film and achieve optimal drug release. In this phase, we determine the polymers, plasticizers, APIs, sweeteners, saliva stimulants, flavorings, colorants, stabilizers, and enhancers based on the desired properties of the multi-layer oral thin film. In addition, we also need to optimize the design of the drug content percentage, surface pH, moisture content and hygroscopicity, folding resistance, swelling index percentage, and the physical state of the drug after being incorporated into the multi-layer film. In the formulation design phase, we focus on polymer selection, controlled release mechanism, etc.
Preparation Method Screening
We select the appropriate casting technology based on the properties of the raw materials to produce a multi-layer oral thin film to ensure uniform drug distribution and consistent film thickness. We do this by applying the formula to the substrate and subsequently drying it to form a thin and flexible film. In addition, we need to make repeated adjustments to get repeatable results.
Quality Control
We implement the most stringent quality control protocols, and at this stage, we conduct various tests focusing on disintegration time, tensile strength, and drug release studies, etc., to evaluate the performance of multi-layer oral thin film.
Manufacture
After the completion of the above development phase, we expand the production process to commercial production, through which we finally deliver satisfactory project development results to our customers.
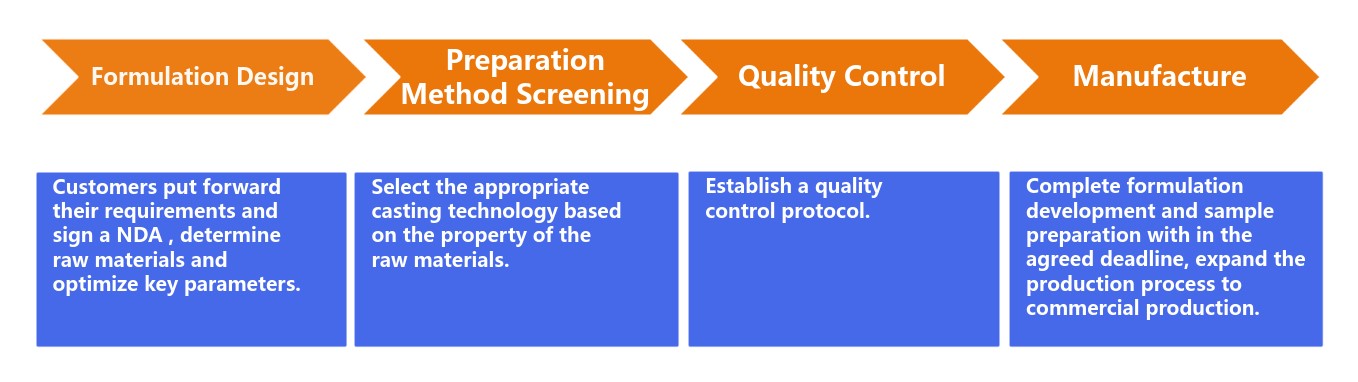 Fig.2 Process of multi-layer oral thin film development. (CD Formulation)
Fig.2 Process of multi-layer oral thin film development. (CD Formulation)
Our Platforms for Multi-Layer Oral Thin Film Formulation Development
| Technologies & Platforms |
Specifics Contents |
| Formulation Design Platform |
We have a professional formula design platform, we determine the polymers, plasticizers, APIs, sweeteners, saliva stimulants, flavorings, colorants, stabilizers, and enhancers based on the desired properties of the multi-layer oral thin film, In the formulation design phase, we focus on polymer selection, controlled release mechanism, etc. |
| X-ray Diffraction (XRD) Analysis Platform |
XRD analysis is a nondestructive technique that provides detailed information about the crystallographic structure, chemical composition, and physical properties of a material. The crystal structure peak of the active substance in the multi-layer oral thin film is observed to provide a basis for the quality control and formulation development of the oral thin films. |
| Differential Scanning Calorimetry (DSC) Analysis Platform |
DSC is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. We study the interaction of active substances with excipients by DSC to elucidate the thermal properties of drugs, thereby predicting any possible physic-chemical interactions that may affect the drug release rate or drug release mechanism of polymer membrane preparations. |
Our Advantages in Multi-Layer Oral Thin Film Development
- Cutting-edge Technology: We have advanced and complete multi-layer oral thin film analysis equipment and production equipment, can choose the most suitable analysis methods and production methods according to the properties of customers' raw materials.
- Complete Development Documentation: Our technicians provide customers with complete development documentation for a multi-layer oral thin film to ensure the repeatability of the development process.
- Customized Solutions: We can provide customized solutions for efficient and patient-friendly multi-layer oral thin film drugs delivery.
- Flexibility: You can ask us about the progress of your project at any time, and we can provide you with current project-related information at any time.
Published Data
Technology: Multilayer oral dispersible film drug delivery technology
Journal: Biochemical Pharmacology
IF: 6
Published: 2022
Results: In this research, we provide at first an overview of the historical background, characterization, and composition of ODFs. Subsequently, technical aspects of the manufacturing process, including three-dimensional printing technology and inkjet printing will be reviewed. As ODFs are promising drug delivery systems for customized small-scale pharmacy preparations, we place particular emphasis on product examples giving the possibility for individualization for their consumers.
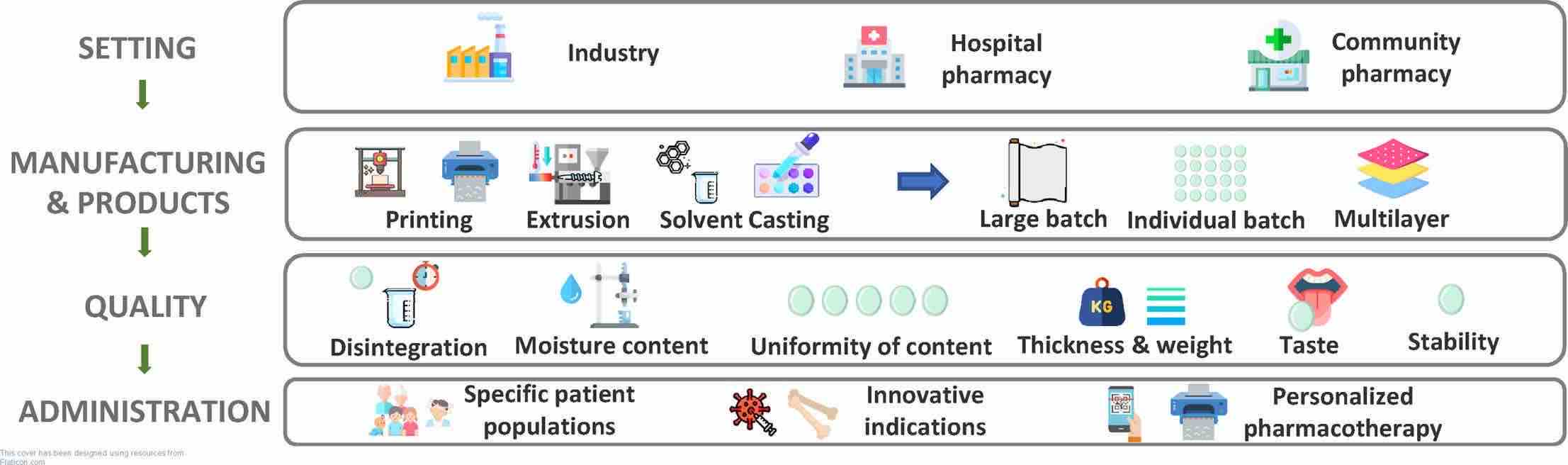 Fig.3 Orodispersible films – Recent developments and new applications in drug delivery and therapy. (B. Morath, et al., 2022)
Fig.3 Orodispersible films – Recent developments and new applications in drug delivery and therapy. (B. Morath, et al., 2022)
CD Formulation constantly overcomes difficulties and explores innovations to provide effective development solutions for multi-layer oral thin film drug delivery systems. If you are interested in our multi-layer oral thin film development services, please don't hesitate to contact us for more information, our colleagues will reply to you within three working days.
References
- Maram Suresh Gupta, Tegginamath Pramod Kumar, et al. Orodispersible Thin Film: A new patient-centered innovation. Journal of Drug Delivery Science and Technology. 2020, Vol (59).
- B. Morath, S. Sauer, et al. Orodispersible films – Recent developments and new applications in drug delivery and therapy. Biochemical Pharmacology. 2022.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services






