Mucoadhesive Sustained-Release Film Development
Inquiry
Mucoadhesive sustained-release film is a promising form of drug that can adhere to the oral mucosa and gradually release the APIs of the drug over some time, and stable distribution in the body and ensuring durable absorption. CD Formulation can support customers in developing mucoadhesive sustained-release film as a new type of drug delivery method, not only maintains the purpose of slow release of drugs but also is used for the presence of dosage forms at the absorption site.
 Fig.1 Mucoadhesive oral films: The potential for unmet needs. (Branca M.A. Silva, et al., 2015)
Fig.1 Mucoadhesive oral films: The potential for unmet needs. (Branca M.A. Silva, et al., 2015)
Mechanisms of Mucoadhesive Sustained-Release Film
The mechanism of the mucoadhesive sustained-release film is generally divided into two steps: the contact stage and the consolidation stage. The first stage is characterized by the contact of the mucoadhesive with the mucus film, and as the formula spreads and expands, the deep contact with the mucus layer begins. In the consolidation step, the bonding materials are activated by the presence of moisture. Moisture plasticizes the system, detaches the adhesive molecules, and connects them by weak van der Waals and hydrogen bonds.
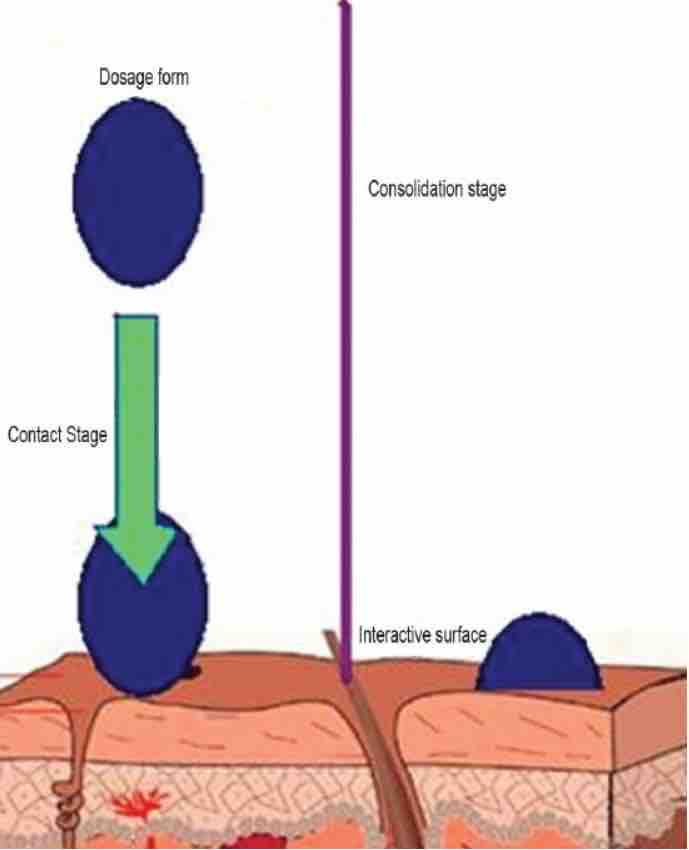 Fig.2 The process of contact and consolidation. (Bindu M, et al ., 2010)
Fig.2 The process of contact and consolidation. (Bindu M, et al ., 2010)
Why Develop Mucoadhesive Sustained-Release Film?
- It avoids the liver first-pass effect and gastrointestinal enzyme degradation.
- It reduces the number of times patients take medication, which helps to improve patient's compliance with the treatment regimen, especially for chronic patients who need to take medication for a long time, minimize side effects, and improve treatment effectiveness.
- It ensures steady release of the drug over time, thereby improving overall efficacy by maximizing absorption and prolonging the duration of action.
- It may be preferred over adhesive tablets in terms of flexibility and comfort. In addition, they can circumvent the relatively short residence time of oral gels on the mucosa, which are easily washed away and removed by saliva.
Our Mucoadhesive Sustained-Release Film Development Services
Formulation Design
During the formulation design phase, our team of experts uses central composite design (CCD) to create various formulations by screening suitable polymers, plasticizers, slow-release materials, mucoadhesive and water, and create the optimal formulation by statistical screening. The 34 factorial methods based on drug content, swelling index, folding endurance, and mucoadhesive strength are used to optimize the formulation. In addition, during the formulation design phase, we also focus on polymer selection, drug-polymer compatibility, and controlled release mechanisms.
Preparation Method Screening
Preparation method screening is critical to the quality of the mucoadhesive sustained-release film, and we are accustomed to using the most advanced casting technology to ensure film thickness uniformity and repeatability.
Quality Control
We implement strict quality control protocols throughout the production process to ensure product consistency. In addition, we utilize drug release kinetics to comprehensively evaluate drug release kinetics to validate the extended-release profile. We use mechanical strength analysis to assess the mechanical strength of the film to ensure its integrity during use. In this phase of safety assessment, we focus on biocompatibility studies and toxicological assessments. We have also developed a fast and reliable in vitro method for differentiating the adhesion strength of various oral films using a texture analyzer.
Manufacture
We have very rich experience in the pilot transfer of the mucoadhesive sustained-release film, and we also have strong manufacturing capabilities.
Our Technologies for Mucoadhesive Sustained-Release Film Development
| Technologies |
Specifics Contents |
| Compatibility Research Platform |
We provide a one-stop service for compatibility during the development of oral dissolved films, including APIs-excipients compatibility, compatibility of two or more APIs, compatibility between excipients, and packaging materials compatibility, etc. |
| Formula Optimization Platform |
We use central composite design (CCD), design of experiments (DOE), orthogonal experimental design, etc., to create various formulations, and get the optimal formula by statistical screening. |
| Process Development Platform |
Our scientists can develop mucoadhesive sustained-release film at laboratory, pilot, and production scales while optimizing process parameters at the early development stage to scale up. We also compare preparation methods to optimize process parameters. |
| Analysis and Characterization Platform |
We can offer multiple analysis methods to confirm the quality of mucoadhesive sustained-release film, including surface pH, thickness, swelling index, disintegration time, mechanical properties, tensile strength, etc. Our analytical laboratory center is equipped with a variety of advanced equipment. |
Our Advantages in Mucoadhesive Sustained-Release Film Development
- Keen Insight: Our team of experts has a keen insight into new drug delivery systems and has an elaborate mucosal adhesion sustained-release drug delivery system, able to achieve sustained release of film drug.
- Complete Experimental Platform: We have a strict drug development environment, high-tech instruments and equipment, and a complete experimental platform. Prepared films were evaluated for their weight, thickness, surface pH, swelling index, drug content uniformity, in vitro residence time, folding endurance in vitro release and permeation studies.
- End-to-End Services: We provide end-to-end services, from pre-formulation research to commercial production, to ensure customers can bring their products to market as quickly as possible.
Published Data
Technology: In vitro release studies were performed using USP XXIV six station dissolution apparatus type 1.
Journal: Indian J Pharm Sci
IF: 1
Published: 2010
Results: The present study concludes that these erodible mucoadhesive buccal films containing enalapril can be very promising for effective doses to systemic circulation. These may also provide an added advantage of circumventing the hepatic first pass metabolism. Films exhibited controlled release over more than 10 h in permeation studies. Further, the study may be extended for assessing the in vivo release and in vitro-in vivo correlation.
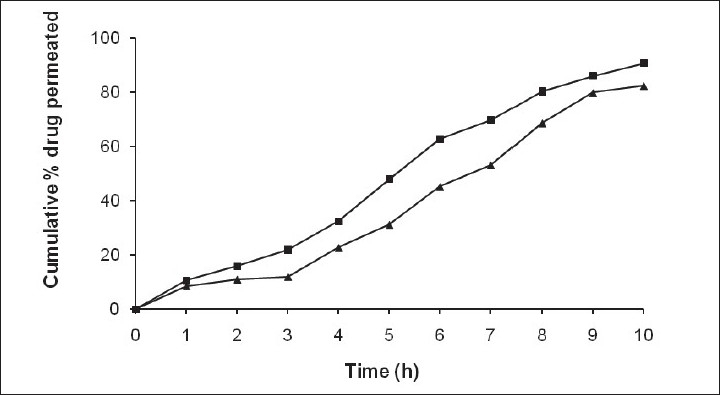 Fig.3 Ex vivo permeation studies of selected mucoadhesive buccal films of enalapril. (A Semalty, et al., 2010)
Fig.3 Ex vivo permeation studies of selected mucoadhesive buccal films of enalapril. (A Semalty, et al., 2010)
CD Formulation is committed to helping customers achieve success in mucoadhesive sustained-release film development and production through innovative technology and high-quality services. If you are interested in our mucoadhesive sustained-release film development services, please don't hesitate to contact us for more information.
References
- Branca M.A.Silva, Ana Filipa Borges, et al. Mucoadhesive oral films: The potential for unmet needs. International Journal of Pharmaceutics. 2015, Vol (494): 537-551.
- Bindu M. Boddupalli, Zulkar N. K. Mohammed, et al. Mucoadhesive drug delivery system: An overview. J Adv Pharm Technol Res. 2010, 1(4):381-387.
- A Semalty, Mona Semalty, et al . Formulation and Evaluation of Mucoadhesive Buccal Films of Enalapril Maleate. Indian J Pharm Sci, 2010, 72(5): 571–575.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services






