One of the most critical parameters for active pharmaceutical ingredients (APIs), excipients, and pharmaceutical products is particle properties, which is an important topic in research and development (R&D), production, and quality control. CD Formulation offers a wide range of particle size analysis services to support the pharmaceutical industry in optimizing their formulations and processes. We utilize advanced techniques such as laser diffraction, dynamic light scattering, and microscopy to accurately measure particle size distribution, shape, and surface area.
The Importance of Particle Size Analysis
Particle size analysis is an important parameter in drug development and manufacturing and plays an important role in determining the safety, effectiveness, and performance of a drug. It not only affects the fluidity, content uniformity, dissolution, etc., of the drug but also affects the surface area and porosity, thereby affecting the bioavailability, effectiveness, and shelf life of the drug. Apart from this, particle size distribution (PSD) is one of the most important parameters to check when evaluating new drugs. In drug research and development, particle size analysis can ensure that the drug meets the prescribed standards and requirements during the preparation process, and optimize the drug formula and production process. Therefore, the particle size of APIs and drug products must be carefully measured and controlled to ensure their quality and performance.
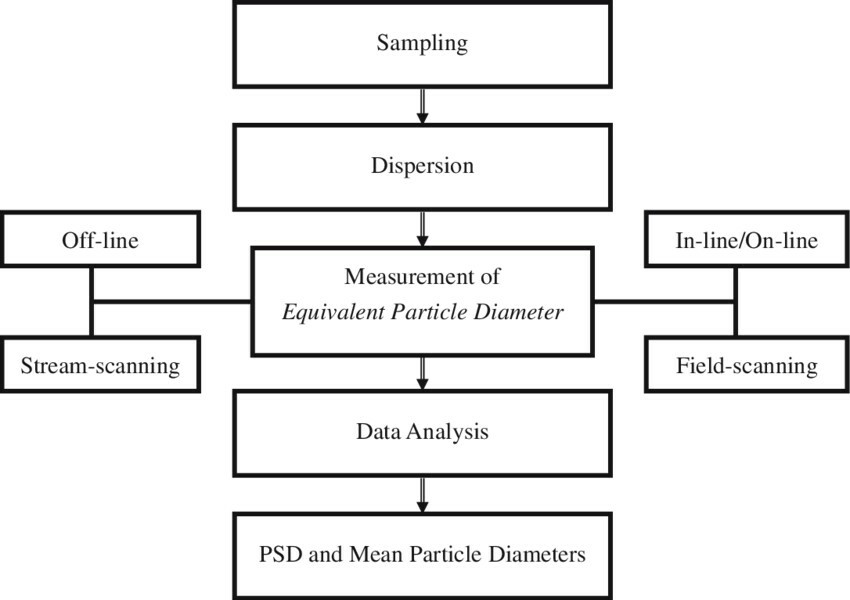 Fig. 1. Methodology of particle size analysis. (Shekunov BY, et al., 2007)
Fig. 1. Methodology of particle size analysis. (Shekunov BY, et al., 2007)
Explore Our Particle Size Analysis Services
As an innovative delivery system development service provider and a global leader in CMC analysis services. CD Formulation provides you with unparalleled particle size analysis services to help you develop safe and reliable pharmaceutical products, whether you are in early development or commercial stages.
Our services include but are not limited to:
- Particle size distribution analysis of solid drug substances, drug products, and excipients using screening techniques.
- Visible and subvisible particle contamination testing.
- Scanning electron microscopy analysis.
- Thermogravimetric analysis (TGA).
- Grain size analysis.
- Analysis of submicron to millimeter ions using Malvern laser diffraction technology.
Available Particle Size Analysis Methods
CD Formulation offers multiple particle size options to support all your possible projects, including but not limited to:
| Methods |
Description |
Sample volume |
Particle size |
| Analytical sieving |
A method of selection for coarse-grained classification of single powders or granules. |
10g-100g |
25 μm - 2,000 μm |
| Dynamic light scattering |
Mainly used to measure the particle size distribution of sub-micron particles. |
0.1 g – 0.5 g |
0.0003μm - 5μm |
| |
Measured using electrophoretic light scattering at a 90 degree scattering angle. |
0.1 g – 0.5 g |
3.8 nm - 100 μm |
| Zeta potential |
Determine the diffusion coefficient of molecules in solution and thus the size of spherical particles through molecular motion. |
~ 100 μg |
~ 0.0005 μm - 0.03 μm |
| Laser light diffraction |
Measured by the dispersion and absorption of laser-generated light (red and blue light) using wet or dry dispersion techniques. |
- Wet dispersion technology: 0.1 g – 0.5 g
- Dry dispersion technology: 0.5 g – 5 g
|
- Wet dispersion technology: 0.01 μm – 600 μm
- Dry dispersion technology: 0.2 μm – 3,500 μm
|
| Quasi-elastic light scattering (QELS) |
A static light scattering technology, which can be used in conjunction with size exclusion chromatography (SEC) as an online detection technique. |
~ 100 μg |
0.01μm - 0.5μm |
Why Choose CD Formulation for Particle Size Analysis?
- A variety of particle sizes from nanometer to micron scale can be analyzed.
- A wide range of testing options and analysis conditions to meet a variety of sample analysis and data reporting needs.
- Flexible, tailor-made solutions.
- Expertise in comprehensive quality assurance in particle size analysis.
- Fast and efficient service ensures that customer projects can be completed on time.
- Ensure the safe release of drugs before market launch.
CD Formulation has extensive experience and expertise in performing particle size analysis services. Our team of experienced scientists will work with you to develop a customized testing plan that meets your specific needs. Please contact us to learn more about how we can help you conduct particle size testing and verify the safety of your products to regulatory requirements.
Reference
- Shekunov BY, Chattopadhyay P, Tong HH, et al. Particle size analysis in pharmaceutics: principles, methods and applications. Pharm Res. 2007, 24(2):203-27.
Please note: Our products and services are not intended to be used directly in diagnostic or therapeutic procedures.
Related Services


 Fig. 1. Methodology of particle size analysis. (Shekunov BY, et al., 2007)
Fig. 1. Methodology of particle size analysis. (Shekunov BY, et al., 2007)