Oral Sustained/Controlled-Release Drug Delivery System Development
InquiryOral sustained/controlled release drug delivery systems are designed to release drugs over extended periods, maintain desired therapeutic concentrations in the blood, reduce dosing frequency, and improve patient compliance. CD Formulation has continuously explored and established an innovative sustained-release oral solid formulation platform, which can achieve sustained-release of drugs through a variety of technologies, such as controlled-release films, fusion preparations, microsphere preparations, etc., to ensure sustained release of drugs and maintain a stable therapeutic concentration in the body.
Why You Need a Sustained/Controlled-Release Drug Delivery System?
Oral drug delivery is the most preferred and convenient option because the oral route provides the largest active surface area among all drug delivery systems used to administer various drugs. However, conventional dosage forms often lead to wide fluctuations in drug concentrations in the bloodstream and tissues, resulting in undesirable toxicity and inefficiency. Sustained and controlled release drug delivery systems (SCRDDS), as an innovative drug delivery system, can use special pharmaceutical preparations or carriers to release drugs at a certain rate. Its core is that it can be slowly released over a long period of time after a single administration and maintain a stable drug level in the blood, thereby achieving the purpose of extending the effect time and reducing toxic and side effects.
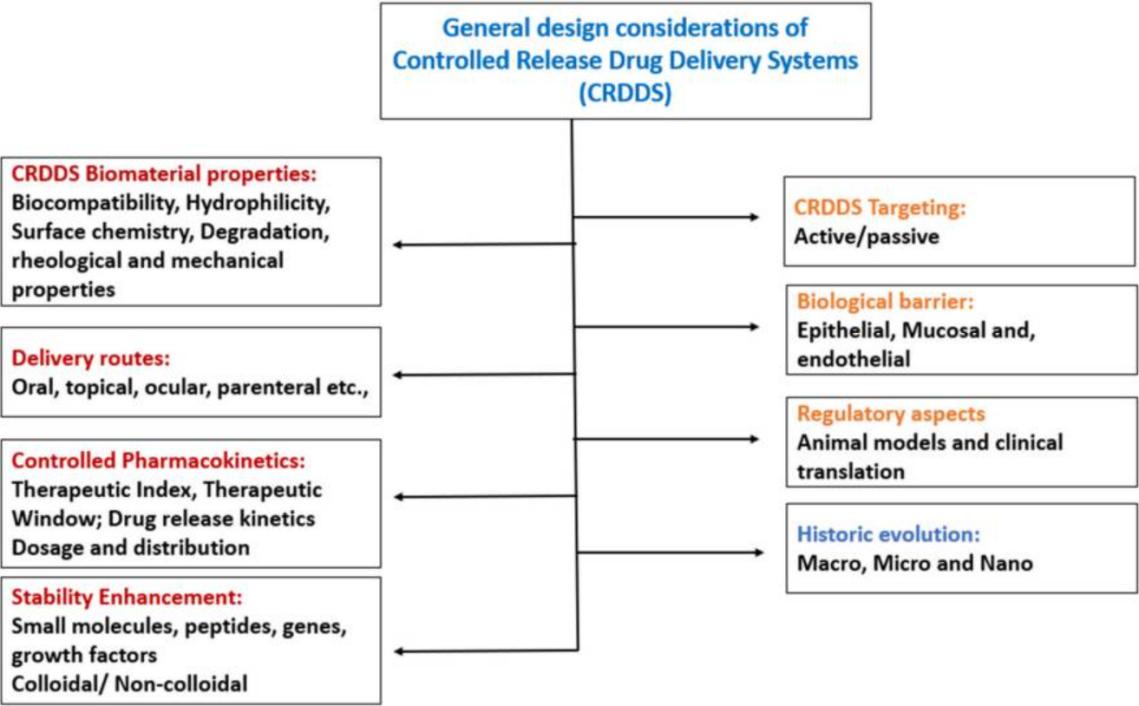 Fig. 1. General design considerations of controlled release drug delivery systems (CRDDSs). (Adepu S, et al., 2021)
Fig. 1. General design considerations of controlled release drug delivery systems (CRDDSs). (Adepu S, et al., 2021)
Advantages Oral Sustained/Controlled-Release Drug Delivery System
- Reduce overdose.
- Improve patient compliance.
- Reduce side effects.
- Improve treatment effect.
- Reduce the frequency of dosing.
Explore Our Oral Sustained/Controlled-Release Drug Delivery System Development Services
CD Formulation is good at improving traditional immediate-release drugs into innovative sustained- and controlled-release drug formulations through our advanced technology platform, including but not limited to poor solubility, poor permeability, and combination drugs. In addition, we can also provide customized CD Formulation services, making personalized adjustments and optimizations based on customer needs and drug attributes to achieve better therapeutic effects and drug stability.
Our services include:
Develop appropriate targeted delivery strategies and protocols.
Design and optimize carrier systems suitable for specific drugs.
Evaluate and verify the effectiveness and safety of delivery systems.
Provide customized solutions and technical support.
What Sustained and Controlled Release Oral Formulations Can We Develop?
- Regular extended-release tablets for daily dosing.
- Multi-unit formulations for extended and controlled release.
- Enteric-coated tablets.
- Gastric suspension tablets.
- Sustained release capsules.
- Enteric coated capsules.
- Orally disintegrating tablets (ODT).
- Modified release (MR) tablets.
- Double layer skeleton tablets
- ...
Our Technical Methods
- Matrix systems. In this method, the drug is dispersed throughout a hydrophilic or hydrophobic matrix material, which controls the release of the drug over a period of time by varying the composition of the matrix material
- Coating systems. In this method, the drug is coated with a polymer or other material that controls the release of the drug. We utilize different coating materials to achieve different release profiles, such as immediate-release, delayed-release, or extended-release.
- Osmotic systems. Osmotic delivery systems use osmotic pressure to control the release of the drug. The drug is contained within a semi-permeable membrane, which allows water to enter the system and create osmotic pressure, pushing the drug out of the system at a controlled rate.
- Microsphere systems. In this method, the drug is encapsulated within microspheres. we can achieve a specific release profile by customizing the size and composition of the microspheres.
- Gastroretentive systems. Various design strategies, such as buoyant systems or mucoadhesive systems, are used to achieve gastroretention, prolonging the residence time of the drug in the stomach or small intestine, and allowing for sustained release of the drug.
Why Choose CD Formulation for Sustained/Controlled-Release Drug Delivery System Development?
- Always at the forefront of innovative drug delivery system development and drug analysis.
- A team of highly skilled scientists and researchers who has extensive experience and expertise in developing sustained and controlled-release drug delivery systems.
- Customized solutions, we work closely with their clients to understand their needs and develop tailored solutions that meet their unique specifications.
- Cutting-edge technology, we stay up-to-date on the latest advancements in the field and continuously strive to improve their techniques and processes by utilizing state-of-the-art technology and tools.
- Comprehensive supporting analysis services support the development of a variety of sustained-release/controlled-release dosage forms, including but not limited to sustained-release tablets, modified-release tablets, orally disintegrating tablets, matrix tablets, sustained-release capsules, etc. u
- Professional technical support and consulting services to help customers achieve their drug research and development goals.
- Cost-effective and competitive pricing.
CD Formulation is at the forefront of the development of oral sustained/controlled-release drug delivery systems. Leveraging cutting-edge technology and unparalleled expertise, we offer a transformative approach to improving the effectiveness of your medicines. Please contact us today to learn how you can take your pharmaceutical formulation development and manufacturing to the next level.
Reference
- Adepu S, Ramakrishna S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules. 2021, 26(19):5905.
Please note: Our products and services are not intended to be used directly in diagnostic or therapeutic procedures.
Related Services

 Fig. 1. General design considerations of controlled release drug delivery systems (CRDDSs). (Adepu S, et al., 2021)
Fig. 1. General design considerations of controlled release drug delivery systems (CRDDSs). (Adepu S, et al., 2021)