Injectable Suspension Emulsion Formulation Development
InquiryInjectable suspension emulsion has the advantages of good stability, slow drug absorption, and long-lasting effects. Thanks to our innovative injectable formulation platform, CD Formulation continues to explore the latest technologies to support the development of injectable suspension emulsions. Our team of experts is dedicated to providing our clients with the highest quality injectable suspension emulsion formulation development services, whether you are looking to develop a new injectable suspension emulsion or improve an existing formulation.
Advantages and Challenges of Injectable Suspension Emulsion
Injectable suspension emulsion, as a long-acting injection, is usually used to slowly release drugs into the body to extend the action time of the drug and reduce the frequency of injections. Injectable suspension emulsions offer many advantages but also present many challenges. In particular, the stability and compatibility of the drug need to be carefully checked when using this type of injection to ensure that the drug can be evenly distributed in the suspension and remain effective for a long time.
- Reduces pain, irritation, and thrombophlebitis.
- Reduce toxicity.
- Improved stability and solubility.
- Targeted drug delivery.
- Physical instability is prone to occur during storage.
- There are limited approved safe emulsifiers.
- Low drug loading, because the drug is usually dissolved in the oil phase whereas the oil phase usually does not exceed 30% in emulsion systems.
- Approved medium-chain fatty acid triglycerides (MCT) and long-chain fatty acid triglycerides (LCT) don't meet the solubility needs of all lipophilic compounds.
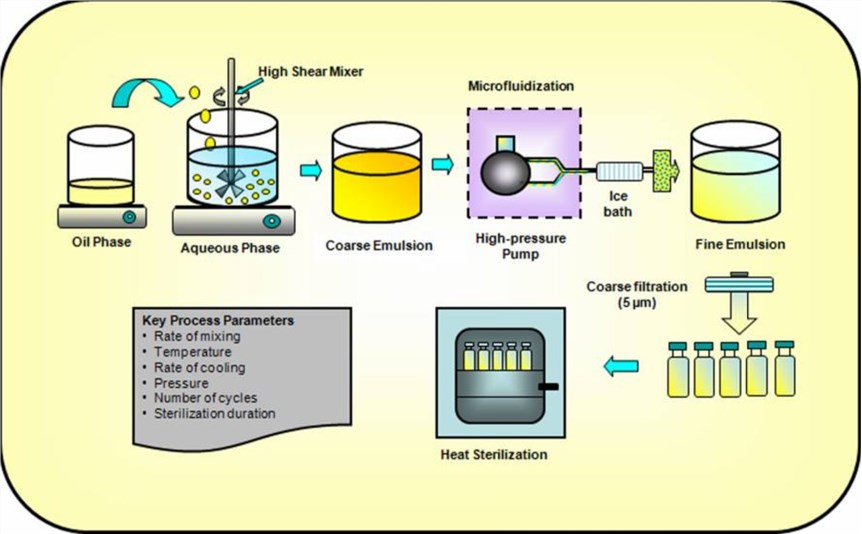 Fig. 1. Key unit operations for preparing injectable lipid emulsions. (Hippalgaonkar K, et al., 2010)
Fig. 1. Key unit operations for preparing injectable lipid emulsions. (Hippalgaonkar K, et al., 2010)
Explore Our Injectable Suspension Emulsion Formulation Development Services
CD Formulation has a proven track record in developing injectables and our team of experts can provide comprehensive support throughout the development process of new injectable pharmaceuticals. Leveraging our unparalleled injectable formulation technology platform, our experienced scientists can help you solve any complex challenge.
Our team of injectable experts continue to explore the latest technologies to support the development of injectable suspension emulsions. For compounds with poor water solubility, nanogrinding technology is used to grind them into nanoscale particles and disperse them evenly in the emulsion.
Importantly, our expert team uses nanotechnology to continuously optimize the emulsion formula. In addition to microemulsions, we can also develop cholesterol-lipid nanoemulsions to enhance the bioavailability of drugs and ensure that the release rate and duration of drugs in the body are consistent with treatment requirements.
Our services include but are not limited to:
- Injectable suspension emulsion formulation development.
- Stability studies.
- Analytical method development and validation.
- Analytical method transfer.
- Drug product release testing.
- Pilot scale scale-up.
Workflow of Injectable Suspension Emulsion Formulation Development
- Preformulation studies: Evaluate the physical, chemical, and mechanical properties of the drug substance to determine its compatibility with the excipients and identify any potential formulation challenges.
- Selection of excipients: Choose excipients based on their functionality, compatibility with the drug substance, and ability to achieve the desired product characteristics.
- Development of formulation: Combine the drug substance with the selected excipients in appropriate ratios and explore various formulation strategies such as emulsification, homogenization, and particle size reduction to optimize the suspension emulsion.
- Characterization of product: Perform physicochemical and mechanical tests on the formulated product to assess its stability, particle size distribution, viscosity, and other critical quality attributes.
- Stability studies: Conduct accelerated and long-term stability studies to evaluate the physical and chemical stability of the formulation under various storage conditions.
- Regulatory considerations: Ensure compliance with regulatory guidelines and requirements for injectable suspension emulsion formulations, including documentation of formulation development activities and submission of relevant data for approval.
- Scale-up and manufacturing: Transfer the optimized formulation to larger-scale manufacturing processes while maintaining product quality and consistency.
- Quality control and assurance: Implement quality control measures such as in-process testing, batch release testing, and ongoing monitoring to ensure the safety, efficacy, and quality of the final product.
Available Analytical Standards
- Uniform milky white or similar color, no precipitation or layering.
- Appropriate pH, usually between 6.0-8.0.
- Sterile and pyrogen-free.
- Transparent or almost free of visible particles.
- For long-term stability testing, there should be no signs of phase separation or aggregate formation.
- Have appropriate particle sizes.
- The precipitates should be easily dispersed after shaking.
Why Choose CD Formulation for Injectable Suspension Emulsion Development?
- Expertise: A team of experienced formulators who have the knowledge and skills to develop injectable formulations and optimize the formulation to ensure stability, compatibility, and efficacy.
- Regulatory compliance: Has a strong understanding of regulatory requirements for injectable formulations and ensures that the formulation meets all regulatory standards and guidelines.
- Customized solution: Work closely with clients to develop customized formulations that meet their specific needs and requirements to ensure optimal drug delivery and bioavailability.
- Advanced technology: Utilize state-of-the-art technology and equipment, allowing for precise control over the formulation process, leading to consistent and reliable results.
- End-to-end capabilities: From pre-formulation research to production of clinical trial drugs.
If you are seeking professional injectable suspension emulsion development services, please contact our team and we will be happy to provide you with support and assistance. Let CD Formulation be your reliable partner in the drug development process.
References
- Hippalgaonkar K, Majumdar S, Kansara V. Injectable lipid emulsions-advancements, opportunities and challenges. AAPS PharmSciTech. 2010, 11(4):1526-40.
- Li Y, Yin H, Wu C, et al. Preparation and in vivo evaluation of an intravenous emulsion loaded with an aprepitant-phospholipid complex. Drug Deliv. 2023, 30(1):2183834.
Please note: Our products and services are not intended to be used directly in diagnostic or therapeutic procedures.
Related Services

 Fig. 1. Key unit operations for preparing injectable lipid emulsions. (Hippalgaonkar K, et al., 2010)
Fig. 1. Key unit operations for preparing injectable lipid emulsions. (Hippalgaonkar K, et al., 2010)