Injectable Nanoparticle Formulation Development
InquiryInjectable nanoformulation is a new way of drug delivery, which improves the solubility, stability, and targeting of the drug by encapsulating the drug in a nanoscale size to achieve better therapeutic effects. As an expert in the field of innovative drug delivery systems, CD Formulation is committed to providing global customers with high-quality injectable nanoparticle formulation development services. Whether you are looking to enhance the solubility of a poorly water-soluble drug, improve the stability of a sensitive drug compound, or achieve targeted delivery to a specific site of action, our team can design a custom injectable nanoparticle formulation that meets your objectives.
Advantages of Nanoinjection
Conventional injections are generally formulated as solutions, suspensions, or emulsions. However, it is difficult to prepare active pharmaceutical ingredients (APIs) with extremely poor water solubility into conventional injections for treatment. Nanotechnology just makes up for this shortcoming and can encapsulate APIs with extremely poor water solubility in nanoparticles, enhance the solubility of drugs that are difficult to dissolve in water, and thereby improve bioavailability. Additionally, nanotechnology can facilitate targeted delivery of drugs to specific organs, tissues, cells, and even organelles. Compared with conventional injections, nanoinjections have the following advantages:
- More precise drug delivery method: involves accurately delivering drugs to specific cells or tissues, improving the bioavailability and efficacy of drugs, reducing the distribution and excretion of drugs in the body, and reducing the toxicity and side effects of drugs on other tissues.
- High drug stability: involves improving the stability and solubility of the drug, extending the half-life of the drug in the body, reducing the metabolism and excretion of the drug, and extending the duration of the drug effect.
- Low toxicity and side effects: involves targeted delivery, reducing drug damage to other organs of the human body, and reducing adverse reactions caused by drugs.
- Long-lasting release: involves achieving controlled release of drugs by adjusting the size and surface properties of nanoparticles, extending the duration of drug effect, and reducing the frequency and frequency of medication.
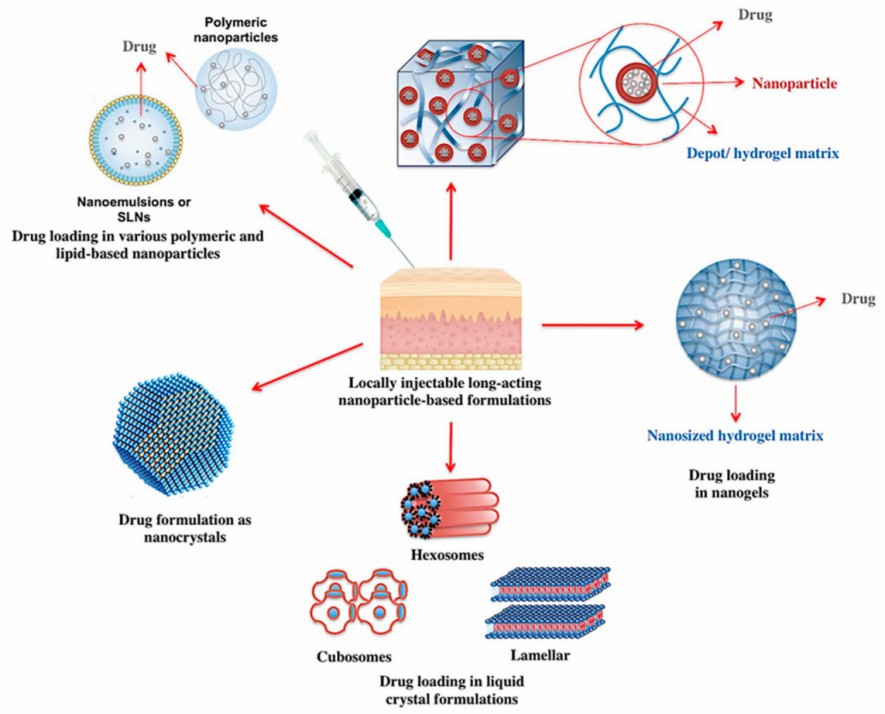 Fig. 1. Different platforms where nanotechnology has contributed to the development of locally injectable long-acting formulations. (Shetab Boushehri MA, et al., 2020)
Fig. 1. Different platforms where nanotechnology has contributed to the development of locally injectable long-acting formulations. (Shetab Boushehri MA, et al., 2020)
Explore Our Injectable Nanoparticle Formulation Services
CD Formulation has a proven track record in developing injectables and our team of experts can provide comprehensive support throughout the development process of new injectable pharmaceuticals. Leveraging our unparalleled injectable formulation technology platform and innovative nanotechnology, our experienced scientists can help you solve any complex challenge.
Our team of nanoformulation experts specializes in developing a variety of injectable nanoformulations, whether for drug delivery, vaccine development, or other medical applications, we can provide customized solutions. Our services include but are not limited to:
- Injectable nanoformulation formulation development.
- Analytical method development and validation.
- Stability studies.
- Preclinical research support.
- Pharmacokinetic studies.
- Scale up from lab to pilot to commercialization.
- Analytical method transfer.
Our Expertise for Injectable Nanoparticle Formulation Development
In addition to conventional injectable evaluation projects, we also provide the following testing services for injectable nanoformulation, including but not limited to:
- Physicochemical parameters of injectable nanoformulation critical quality attributes(CQAs) were evaluated, including shape and surface morphology, particle size, zeta potential, and API-excipient compatibility.
- Shape and surface morphology: Scanning electron microscopy (SEM).
- Particle size and size distribution: Zeta sizer, photon correlation spectroscopy (PCS).
- Zeta potential measurement: Zeta sizer.
- API-excipient compatibility: Fourier transform infrared (FTIR) analysis.
- Encapsulation efficiency assessment.
- Drug loading assessment.
- In vitro release assessment.
- Stability assessment.
- Hemolysis testing.
- Residual solvent testing (the manufacturing process of injectable nanoformulation usually uses certain organic solvents for synthesis or encapsulation).
Why Choose CD Formulation for Injectable Nanoparticle Formulation Development?
- Expertise: A team of experienced formulators who have the knowledge and skills to develop injectable formulations and optimize the formulation to ensure stability, compatibility, and efficacy.
- Regulatory compliance: Has a strong understanding of regulatory requirements for injectable formulations and ensures that the formulation meets all regulatory standards and guidelines.
- Customized solution: Work closely with clients to develop customized formulations that meet their specific needs and requirements to ensure optimal drug delivery and bioavailability.
- Advanced technology: Utilize state-of-the-art technology and equipment, allowing for precise control over the formulation process, leading to consistent and reliable results.
- End-to-end capabilities: From pre-formulation research to production of clinical trial drugs.
If you are seeking professional injectable nanoparticle formulation services, please contact our team and we will be happy to provide you with support and assistance. Let CD Formulation be your reliable partner in the drug development process.
Reference
- Shetab Boushehri MA, Dietrich D, Lamprecht A. Nanotechnology as a Platform for the Development of Injectable Parenteral Formulations: A Comprehensive Review of the Know-Hows and State of the Art. Pharmaceutics. 2020, 12(6):510.
Please note: Our products and services are not intended to be used directly in diagnostic or therapeutic procedures.
Related Services

 Fig. 1. Different platforms where nanotechnology has contributed to the development of locally injectable long-acting formulations. (Shetab Boushehri MA, et al., 2020)
Fig. 1. Different platforms where nanotechnology has contributed to the development of locally injectable long-acting formulations. (Shetab Boushehri MA, et al., 2020)