The rate at which an active pharmaceutical ingredient (API) is released from its formulation depends on a variety of factors, including the chemical nature of the drug, the nature of the formulation, the method of preparation, the carrier used, and the release mechanism. CD Formulation provides unparalleled in-vitro release testing (IVRT) services to our global clients in drug development, helping with formulation optimization, quality control, bioequivalence evaluation (BE) of generic drugs, and regulatory submissions.
The Importance of In-Vitro Release Testing
In vitro release testing (IVRT), a critical step in drug product development and evaluation, involves studying the release of an API from a dosage form (such as a tablet, capsule, or cream) into a suitable medium in a controlled laboratory environment. The results of IVRT provide valuable information about drug release characteristics, including release rate and extent, which can help:
- Quality control: Evaluate the performance of different formulations under standardized conditions to identify any potential issues with formulation variations, manufacturing processes, or stability over time.
- Regulatory compliance: Regulatory agencies such as the US FDA require manufacturers to conduct IVRT to prove the safety, effectiveness, and quality of their products.
- Drug formula optimization: Help optimize drug formulas to achieve the required release profile and therapeutic effect, and develop more effective and efficient drugs by studying factors affecting drug releases, such as dosage form type, excipients, and manufacturing processes.
- Predict in vivo performance: Provide valuable insights into the in vivo performance of drug products by simulating drug release in a controlled laboratory environment.
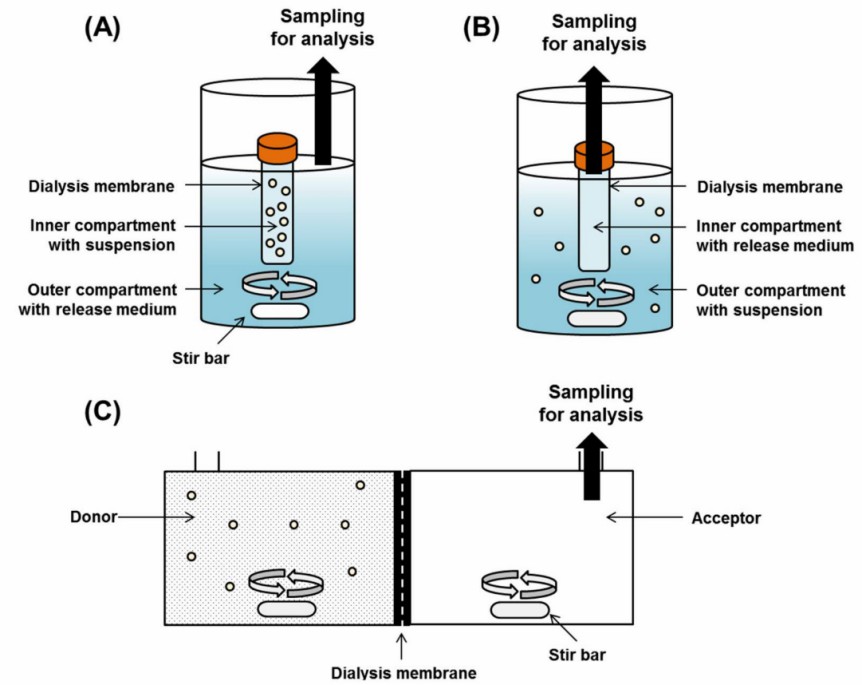 Fig. 1. Dialysis membrane methods for in vitro drug release test of particulate formulations: regular dialysis (A), reverse dialysis (B), and side-by-side dialysis (C). (Kim Y, et al., 2021)
Fig. 1. Dialysis membrane methods for in vitro drug release test of particulate formulations: regular dialysis (A), reverse dialysis (B), and side-by-side dialysis (C). (Kim Y, et al., 2021)
Explore Our In-Vitro Release Testing Services
As an expert in the development of innovative drug delivery systems and a global leader in CMC analysis, we provide unparalleled in-vitro release testing (IVRT) services to our global clients in drug development, helping them bring their products to market quickly. Our IVRT services evaluate the rate and extent of drug release in the body by simulating IVRT to ensure the effectiveness and stability of the drug delivery system.
Our team of IVRT experts have the ability to perform the following, including but not limited to:
- Development and validation of new TVRT analysis methods.
- Adaptation and optimization of existing IVRT methods.
- Conduct IVRT experiments and product testing according to GLP guidelines.
- BE of generic drugs.
- Support scale-up for pharmaceutical product development.
Our Expertise for In-Vitro Release Testing
- For semi-solid formulations, we use a Franz diffusion cell based on a vertical diffusion cell and select synthetic membranes, tissue constructs, or biological samples to form a diffusion system for IVRT based on the characteristics of your sample.
- For liquid preparations, such as emulsions, we usually use the shaker method, placing the sample into a container containing buffered salt medium (usually artificial gastric juice or artificial intestinal juice), keeping it closed at a constant temperature, and after a certain period of time, samples are taken and measured by HPLC or UPLC and replenish the medium with fresh buffered salt.
- For solid preparations, they are usually dissolved first, and then the sample is placed in a container containing a buffered salt medium (usually artificial gastric juice or artificial intestinal juice) and kept closed at a constant temperature. After a certain period of time, samples are taken and measured by HPLC or UPLC, and replenish the medium with fresh buffered salt.
- For nanopreparations, we usually use the sample and separate (SS) method, dialysis membrane (DM) method, and continuous flow (CF) method for measurement.
- In the SS method, the nanoformulation is placed in a release medium maintained at a constant temperature, and drug release is assessed by sampling the release medium (filtrate or supernatant) or the nanoformulation.
- In DM, the nanoformulation is placed into a dialysis bag containing the release medium (inner medium/compartment), which is subsequently sealed and placed in a larger container containing the release medium (outer medium/compartment) while stirring. During this process, keep it closed and at a constant temperature, take samples for measurement by HPLC or UPLC after a certain period, and add a fresh release medium.
- In the CF method, we usually use the United States Pharmacopeia (USP) apparatus IV for IVRT.
In Vitro Penetration Testing (IVPT) Services
In addition to IVRT services, we also provide in-vitro permeability testing (IVPT) services for semi-solid preparations for transdermal administration. Our IVPT services are also based on the Franz diffusion cell of a vertical diffusion cell.
The difference is that we choose a diffusion system that is made up of biological samples (usually animal skin) at this time. Our IVPT services include but are not limited to:
- Development and validation of new TVPT analysis methods.
- Adaptation and optimization of existing IVPT methods.
- Skin integrity testing.
- BE of generic drugs.
Why Choose CD Formulation for In-Vitro Release Testing?
- Flexible, tailor-made solutions to meet customers' specific needs and requirements.
- Extensive expertise and experience in performing IVRT and IVPT analyses.
- Advanced equipment and technology can test any pharmaceutical dosage form, including sustained-release and nanopreparations.
- Strict quality assurance protocols ensure that all analyses are performed to the highest standards and comply with industry standards.
- Fast and efficient service ensures that customers' projects can be delivered on time.
CD Formulation has extensive experience and expertise in performing IVRT and IVPT analysis services. Our team of experienced scientists will work with you to develop a customized testing plan that meets your specific needs. Please contact us to learn more about how we can help you conduct IVRT and IVPT analyses and verify the safety of your products to regulatory requirements.
References
- Shah VP, Simona Miron D, Ștefan Rădulescu F, et al. In vitro release test (IVRT): Principles and applications. Int J Pharm. 2022, 626:122159.
- Kim Y, Park EJ, Kim TW, et al. Recent Progress in Drug Release Testing Methods of Biopolymeric Particulate System. Pharmaceutics. 2021, 13(8):1313.
Please note: Our products and services are not intended to be used directly in diagnostic or therapeutic procedures.
Related Services

 Fig. 1. Dialysis membrane methods for in vitro drug release test of particulate formulations: regular dialysis (A), reverse dialysis (B), and side-by-side dialysis (C). (Kim Y, et al., 2021)
Fig. 1. Dialysis membrane methods for in vitro drug release test of particulate formulations: regular dialysis (A), reverse dialysis (B), and side-by-side dialysis (C). (Kim Y, et al., 2021)