Solid Lipid Nanoparticle Development for Nanomedicine
Inquiry
CD Formulation can provide our clients with formulation development services for solid lipid nanoparticles (SLNs) for nanomedicines to meet the different requirements using our advanced solid lipid nanoparticle fabrication technology. Our highly qualified nanoformulation development team can design and configure solid lipid nanoparticles based on different loads, thus promoting research and even commercialization of SLNs for nanomedicine.
Advantages of Solid Lipid Nanoparticles as Drug Carriers
SLNs are the first generation of lipid nanoparticles, which are composed of solid natural or synthetic lipid-encapsulated drugs to form a solid colloid drug delivery system. As a new type of submicron colloid drug delivery systems are used as the matrix, and the drug is wrapped in a lipid core to form a solid lipid particle drug delivery system with a particle size of about 50 to 1,000 nm. SLNs combine the advantages of various colloidal systems such as liposomes, nanoemulsions, and polymeric nanoparticles. SLNs exhibit specific characteristics such as high cellular uptake, large surface area, prolonged and controlled drug release, and low toxicity than traditional colloidal carriers like liposomes and emulsions.
- No biotoxic
- No organic solvents during the preparation process
- High physical stability
- Support drug targeting and controlled drug release.
- Active compounds can be encapsulated in SLNs.
- Mass production is simple.
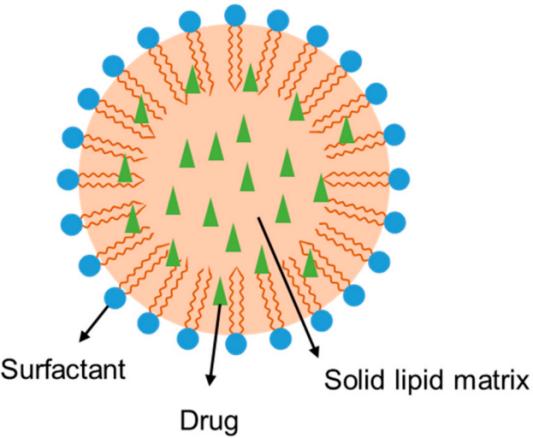 Fig.1 Structure of solid lipid nanoparticles (SLNs). (Dhiman N, et al., 2022)
Fig.1 Structure of solid lipid nanoparticles (SLNs). (Dhiman N, et al., 2022)
What Can We Provide for Solid Lipid Nanoparticle Development?
CD Formulation, as a leading pharmaceutical laboratory services company, provides you with comprehensive, innovative, and timely solutions and helps you quickly develop the formulation of high-quality solid lipid nanoparticles for nanomedicine. We support all phases of the formulation development and even commercialization process of SLNs for nanomedicines through our extensive technical expertise in the SLN technology.
- SLNs formulation design/ fabrication/optimization formulation/ modification.
- Analysis and characterization of SLNs for nanomedicine.
Explore Our Methods for Solid Lipid Nanoparticle Development
There are various methods for preparing SLNs, including thin film-ultrasonic dispersion method, emulsification evaporation-low temperature solidification method, high-pressure emulsion homogenization method, double emulsion-solvent diffusion method, hot melt ultrasonic method, solvent emulsification-ultrasonic method, microemulsion method, etc. A series of preparation measures. CD Formulation relies on our advanced design concept to use different preparation methods according to different nanomedicine properties to prepare SLNs that meet your requirements. We can provide you with the following approaches to the formulation for SLNs as follows:
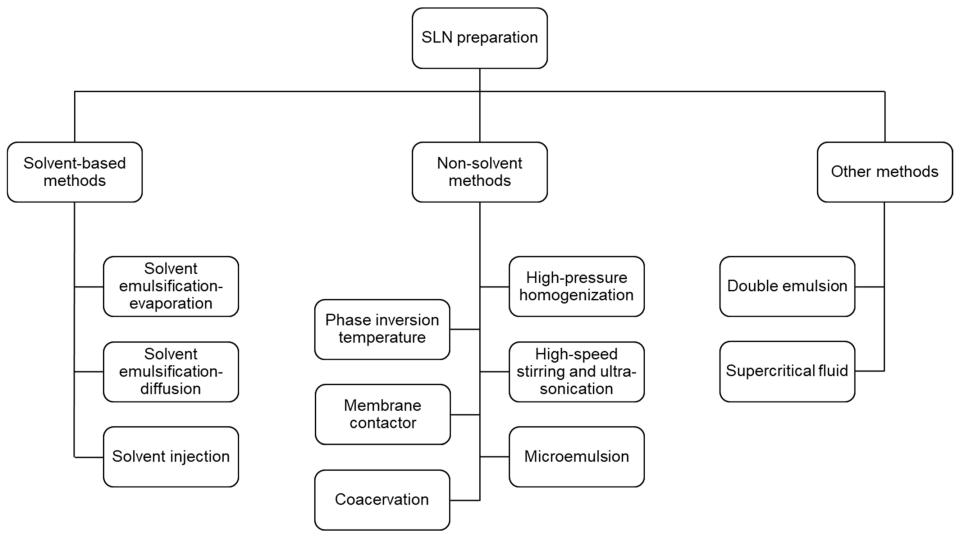 Fig.2 Classification of SLN preparation methods. (Nguyen TTL, et al., 2022)
Fig.2 Classification of SLN preparation methods. (Nguyen TTL, et al., 2022)
High Energy Approaches
- High-Pressure Homogenization (HPH) technique(hot/cold)
This method involves subjecting a lipid dispersion to high pressure to reduce the particle size and create uniform, stable nanoparticles.
- In the hot HPH process, the lipid dispersion is heated to a certain temperature before being subjected to high pressure, to result in smaller particle sizes and improved encapsulation efficiency.
- In the cold HPH process, the lipid dispersion is kept at a low temperature before undergoing high-pressure homogenization, thus creating more stable nanoparticles with better drug-loading capacity.
- High shear homogenization and/or ultrasonication technique
Lipid nanoparticle dispersions are obtained by dispersing the melted lipid in the warm aqueous phase containing surfactants by high sheer homogenization followed by ultrasonication.
Low Energy Approaches
In this technique, the microemulsion is spontaneously formed due to the high surfactants/lipid Ratio. Specifically, the method involves mixing the drug and lipid at a temperature above the melting point of the lipid, preheating an aqueous phase containing surfactant to the same temperature, and adding the aqueous phase to the lipid phase with gentle stirring to form a microemulsion, which is then poured into a cold aqueous solution with mechanical stirring to precipitate to form SLN.
- Membrane contractor technique
In this technique, a lipid solution containing the drug of interest is dispersed in an aqueous phase containing a surfactant. The two phases are then mixed under high shear to form a coarse emulsion. A membrane contractor device is used to disrupt the coarse emulsion and produce smaller droplets of the lipid solution in the aqueous phase, resulting in the formation of SLNs.
- Phase inversion temperature (PIT) technique
This technique mainly depends on the change in the properties of polyoxyethylated surfactants at different temperatures. In this method, lipid, drug, water and surfactant are mixed together on magnetic stirring, three heating and cooling cycles are performed which are then diluted with cold water causing phase inversion of the emulsion and breaking resulting in SLNs.
Lipid nanoparticles are produced by acidification of a micellar solution of fatty acid alkaline salts.
- Double emulsion technique
The double emulsion technique in the preparation of SLNs is suitable for hydrophilic active pharmaceutical ingredients and peptides. In this method, an aqueous solution of the drug is emulsified in melted lipid blend to form primary W/O emulsion stabilized with suitable excipients. This emulsion then is dispersed in the aqueous phase to form a water/oil/water double emulsion and cooled to form SLN.
Solvent-Based Methods
- Emulsification-solvent evaporation technique
The method involves dissolving the lipid and drug in a solvent or solvent mixture to form an oil phase, which is subsequently emulsified in an aqueous phase to form an oil-in-water nanoemulsion. Subsequent evaporation of the solvent results in lipid precipitation and formation of SLNs.
- Emulsification solvent diffusion technique
The method involves the use of organic solvents to generate water-saturated solvent and solvent-saturated water, followed by dissolving the drug and lipids in the solvent and then emulsifying the two phases to form an oil/water emulsion. The emulsion is then diluted with water and dried under a vacuum to eliminate the solvent to form SLN.
- Solvent injection technique
The method includes three steps: (i) preparation of aqueous and oil phases, (ii) solvent injection, and (iii) solvent removal, which involves lipids dissolved in a water-miscible solvent (e.g. acetone, isopropanol, and methanol) or water-miscible solvent mixture and quickly injected into an aqueous solution of surfactants through an injection needle.
- Super critical fluid technique
The method involves the addition of supercritical CO2 to the organic phase before injecting the solvent into the aqueous phase, whereby upon injection, the lipids rapidly precipitate to form SLNs. It has the advantages of highly uniform particle size distribution and high solvent extraction efficiency.
We have broad-based and professional knowledgeable resources and a fully equipped laboratory that retains intellectual property for our clients and the flexibility to meet your specific requirements. In addition to the above-mentioned special approaches, we can also provide you with other additional techniques for the fabrication of SLNs for nanomedicine that you require.
Our Analytical Capabilities for Solid Lipid Nanoparticles
The physical stability of the SLNs during prolonged storage can be determined by monitoring changes in particle size, zeta potential, drug content, appearance, and viscosity as a function of time. CD Formulation can provide you with the following analysis and characterization of SLNs including:
- Particle Size
- Zeta Potential
- Encapsulation Efficiency
- Drug Loading
- Particle Concentration
- Drug Release from Solid Lipid Nanoparticles
- Stability
Why Choose Us to Develop Solid Lipid Nanoparticles?
- We have a leading solid lipid nanoparticle technology platform and can design and structure high-quality solid lipid nanoparticles to meet customers' various needs.
- We can prepare solid lipid nanoparticles through different methods including HPH (hot/cold), emulsification-solvent evaporation, emulsification solvent diffusion, supercritical fluid, coacervation, etc.
- We hold the advanced instruments and equipment and are also able to provide various analysis and characterization of SLNs covering particle size, zeta potential, encapsulation efficiency, drug loading, etc.
- We have a strong and elite team of experts, and they can provide a quick response to your requirements for the SLNs formulation development for nanomedicine immediately.
Published Data
Technology: Solid lipid nanocarriers evaluation using imaging and radiotracer techniques
Journal: Acta Pharmaceutica Sinica B
IF: 14.5
Published: 2021
Results:
The authors review the advantages of SLNs and their applications in oral and pulmonary drug delivery as well as the biological fate of these lipid nanocarriers in vivo. Among different lipid nanocarriers, ISAsomes (internally self-assembled particles or particles) including cubes and hexasomes as well as solid lipid nanoparticles (SLN) have unique structural features that make them attractive as drug delivery nanocarriers. Meanwhile, the authors reported the application of imaging and radiotracer techniques to evaluate the in vivo biodistribution of solid lipid drug nanocarriers, i.e., drug-loaded SLNs from cellular uptake to oral administration. Imaging and radiotracer techniques provide the necessary technical support to assess the in vivo biodistribution of drug-loaded SLNs from cellular uptake to oral administration.
CD Formulation can help global researchers to move solid lipid nanoparticle research to a new level depending on our advanced solid lipid nanoparticle technology platform and strong drug design capabilities. If you are interested in our SLN development services, please do not hesitate to contact us for in-depth discussions.
References
- Dhiman N, Awasthi R, Sharma B, et al. Lipid Nanoparticles as Carriers for Bioactive Delivery. Front Chem. 2021, 9:580118.
- Nguyen TTL, Duong VA. Solid Lipid Nanoparticles. Encyclopedia. 2022, 2(2):952-973.
- Anan Yaghmur, Huiling Mu. Recent advances in drug delivery applications of cubosomes, hexosomes, and solid lipid nanoparticles. Acta Pharmaceutica Sinica B. 2021,11(4):871-885.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 Structure of solid lipid nanoparticles (SLNs). (Dhiman N, et al., 2022)
Fig.1 Structure of solid lipid nanoparticles (SLNs). (Dhiman N, et al., 2022) Fig.2 Classification of SLN preparation methods. (Nguyen TTL, et al., 2022)
Fig.2 Classification of SLN preparation methods. (Nguyen TTL, et al., 2022)
