Nanoformulation Pilot Scale-up Services
Inquiry
Based on our strong expertise and state-of-the-art hardware equipment, CD Formulation is capable of customizing and manufacturing various types of nanomedicines, including polymeric nanoparticles, solid lipid nanoparticles (SLNs), liposomes, dendrimers, polymeric micelles, nanogels, nanofibers, etc. These nanomedicines will be conducive to supporting the clinical research and then promoting their commercial production after pilot scale-up research.
Why are Nanoformulation Pilot Scale-up Services Important?
The success of any nanomedicine depends on its large-scale industrial production and commercialization. Therefore, we pay more attention to large-scale industrial production after the successful development at the laboratory scale. Once the pilot scale-up and even industrial production are achieved, nanomedicines will be better used in the market to treat various diseases. However, any new nanoformulations need to go through the entire process from nanoformulation design, laboratory-scale development to scale-up production before they can be launched on the market.
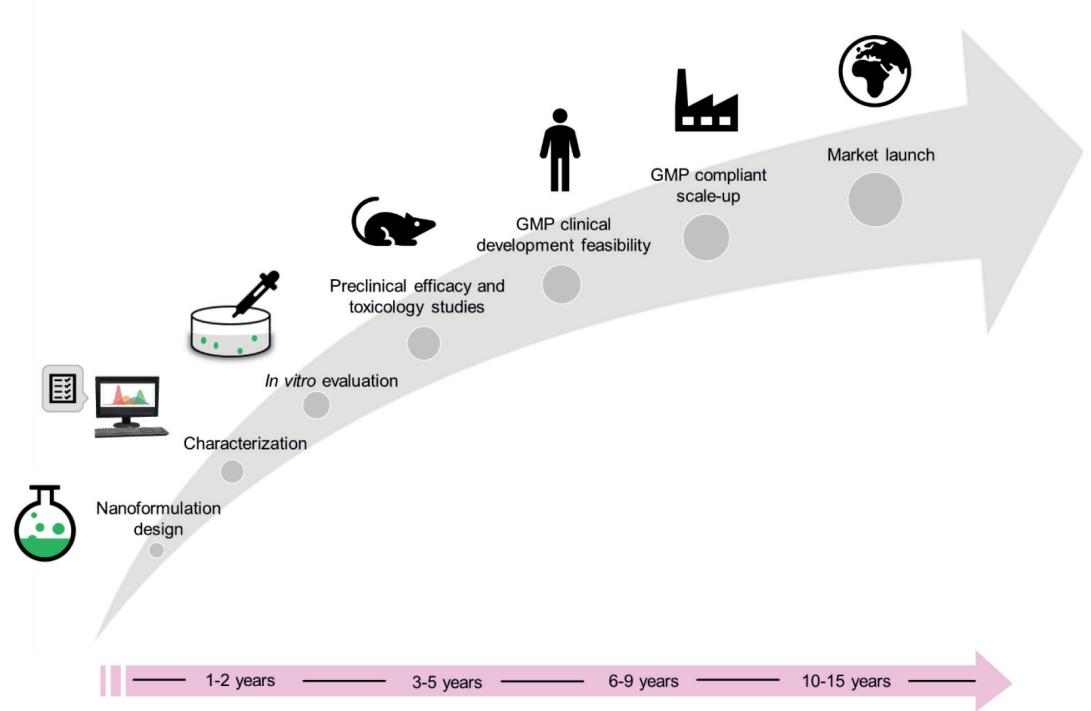 Fig.1 Steps and average timelines for clinical development of nanomedicine formulations. (Maria Camilla Opert, et al. 2023)
Fig.1 Steps and average timelines for clinical development of nanomedicine formulations. (Maria Camilla Opert, et al. 2023)
Although the process of small-scale preparation can be opened up and nanoformulations with expected quality requirements can be obtained, its process and equipment limitations may make it impossible to convert any preparation method from laboratory scale to industrial scale. Thus, our pilot scale-up of nanomedicines involves integration of preparation methods and technology transfer for their large-scale industrial production.
Based on the above clarification, our carefully designed pilot scale-up procedure is a transitional stage from small-scale to commercial production, mainly involving the integration of preparation methods, the conversion of equipment parameters, the optimization of process design space, and product quality control at this stage, which will ensure the quality, cost-effectiveness, and timely product launch of nanomedicines.
Our Solutions for the Pilot Scale-up Nanoformulations
Our pilot scale-up studies have improved the quality of the final nanoformulation and extended the shelf life of the nanoformulation by improving key material parameters and critical process parameters. CD Formulation is committed to exploring and researching the challenges and solutions of nanoformulation pilot scale-up for many years.
Our Pilot Scale-up Challenges in Nanoformulation Production
Amplification effects and process limitations of preparation methods are major challenges during our pilot scale-up production.
- Through pilot experiments, we have observed that amplification affects nanoparticle properties such as particle size, drug entrapment, process residual materials, colloidal stability, and surface morphology.
- We have also understood the process limitations, which mainly include the stability of the materials used in the method and the use of toxic solvents (such as the use of chloroform or dichloromethane as the organic phase).
Our Strategies for the Pilot Scale-up Production
Based on our novel and unique concept of quality by design (QbD), we have established the quality target product profile (QTPP) and critical quality attributes (CQAs) for this nanoformulation formulation to achieve its safety and efficacy goals.
- In the design of QTPPs and CQAs, we have identified particle size, size distribution, surface charge and morphology, drug release, impurity levels, etc. to establish specifications and determine whether the manufacturing process can provide batches that meet the release specifications.
- In production, based on the QbD model, we have focused on the impact of critical process parameters (CPPs) and critical material attributes (CMAs) on critical quality attributes (CQAs) to ensure the quality of the final product.
- To estimate the interaction of these parameters and better understand the process, we have applied a design of experiments (DOE) approach to nanoformulation development to determine the relationship between factors affecting a process and the outcomes of that process, thereby guiding our analysis of the final subsequent optimization of product needs.
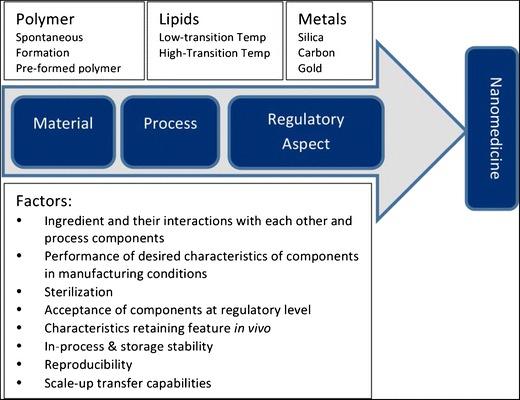 Fig.2 Schematic representation of material and process parameters and regulatory aspects in the development of nanomedicine. (Rishi Paliwal, et al. 2014)
Fig.2 Schematic representation of material and process parameters and regulatory aspects in the development of nanomedicine. (Rishi Paliwal, et al. 2014)
Our Methods for Nanoformulation Pilot Scale-up
Our technical team has conducted an in-depth study of different types of nanoformulation pilot scale-up processes, including but not limited to adjusting the size of equipment and containers, increasing the number of production batches, implementing new quality control measures, optimizing manufacturing processes, and ensuring regulatory compliance guidelines. And we are able to offer nanoformulation pilot scale-up services to meet customers' different requirements. And you can choose the following methods according to your specific requirements to complete your pilot scale-up study.
Work With Experienced Partners
CD Formulation has extensive experience in pilot scale-up and conversion to commercial-scale production and can provide you with valuable assistance in planning and executing your processes.
Develop a Regulatory Compliance Plan
Regulatory compliance is a critical part of scaling up. We can help you plan early in the process to avoid delays or issues.
Establish Strong Quality Control Measures for Nanoformulations
Quality control of nanoformulations is critical at every stage of nanoformulation development, and it is even more important during pilot-scale production. We have lots of experience in nanoformulation pilot scale-up and are able to assist you in establishing strong quality control measures to ensure that the final product meets all regulatory requirements.
Focus on Process Development and Validation
The key part of pilot scale-up is to develop and validate the nanoformulation manufacturing process. Our validation goals are to confirm process parameters, establish critical control points, and test process effects, catering to your personalized requirements.
Why Choose Us for Nanoformulation Pilot Scale-up?
- Relying on our advanced concept of nanoformulation pilot scale-up design and nanotechnology, we are able to provide customers with nanoformulation pilot scale-up services, including designing the pilot scale-up proposal, confirmation and optimization of process parameters, and quality control.
- Our core technical team has rich experience in pilot scale-up and conversion to commercial-scale production. As your true partner, we can help you develop a regulatory compliance plan and establish strong quality control measures to promote the feasibility of pilot scale-up.
- We are also keen on the process development and validation of various nanoformulations, including nanoparticles, liposomes, exosomes, nanogels, nanoemulsions, nanofibers, etc.
Published Data
Technology: Cross flow filtration
Journal: International Journal of Pharmaceutics
IF: 5.3
Published: 2021
Results:
The authors investigated the scale-up process of two chitosan derivatives, methoxypolyethylene glycol-chitosan (mPEG-CS) and methoxypolyethylene glycol-chitosan-oleic acid (mPEG-CS-OA), produced at laboratory scale for use in the pharmaceutical industry using cross flow filtration (CFF) technology. When they produced in larger batches, the two copolymers were able to maintain their physicochemical properties while reducing production time and increasing yield. The authors also demonstrated that neither chitosan derivative exhibited in vitro cytotoxicity independent of the production method. Furthermore, the authors tested the storage stability of polymeric micelles produced from mPEG-CS-OA after scale-up. This study demonstrates the feasibility of cross-flow filtration for the production of polymers and polymer micelles suitable for GMP production.
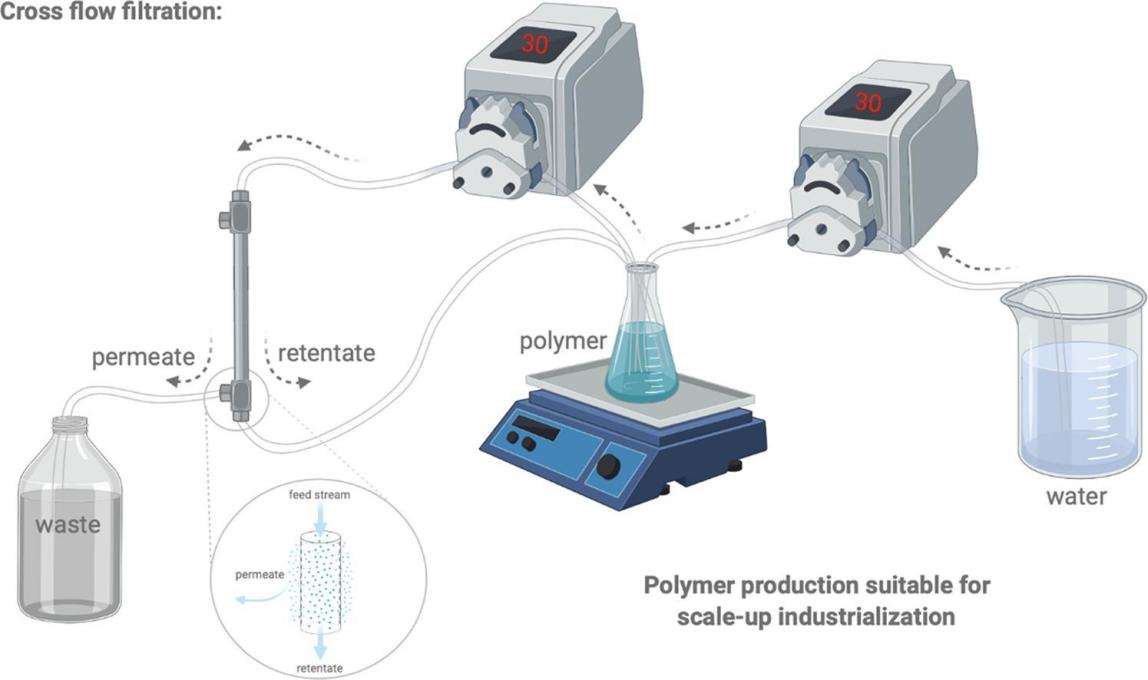 Fig.3 CFF for polymer production suitable for scale-up industrialization. (Junyi Chen, et al. 2020)
Fig.3 CFF for polymer production suitable for scale-up industrialization. (Junyi Chen, et al. 2020)
Pilot scale-up studies are the stage at which nanoformulations are produced at large scale for clinical trials and eventual commercialization. The seamless connection between pilot scale-up research and industrial production of nanoformulations is crucial to ensure the safety of clinical drugs and promote commercial production. CD Formulation, as a nanoformulation R&D service company, can offer our professional nanoformulation pilot scale-up services. If you have any questions on nanoformulation pilot scale-up, please feel free to contact us.
References
- Maria Camilla Opert. Scale-up Manufacturing of Polymeric Immunomodulatory Nanomedicines: from Bench to the Bedside. Radboud Repository. 2023, https://hdl.handle.net/2066/289610.
- Rishi Paliwal, R Jayachandra Babu, Srinath Palakurthi. Nanomedicine scale-up technologies: feasibilities and challenges. AAPS PharmSciTech. 2014, 15, 1527-1534.
- Andreia Almeida, Nazende Günday-Türeli, Bruno Sarmento. A scale-up strategy for the synthesis of chitosan derivatives used in micellar nanomedicines. International Journal of Pharmaceutics. 2021,609,121151.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 Steps and average timelines for clinical development of nanomedicine formulations. (Maria Camilla Opert, et al. 2023)
Fig.1 Steps and average timelines for clinical development of nanomedicine formulations. (Maria Camilla Opert, et al. 2023)  Fig.2 Schematic representation of material and process parameters and regulatory aspects in the development of nanomedicine. (Rishi Paliwal, et al. 2014)
Fig.2 Schematic representation of material and process parameters and regulatory aspects in the development of nanomedicine. (Rishi Paliwal, et al. 2014)  Fig.3 CFF for polymer production suitable for scale-up industrialization. (Junyi Chen, et al. 2020)
Fig.3 CFF for polymer production suitable for scale-up industrialization. (Junyi Chen, et al. 2020) 
