Nanomedicine - CD Formulation
Call Us:
- Home
- Services
- Nanoformulation Development
- Nanoparticle Development for Nanomedicine
- Solid Lipid Nanoparticle Development for Nanomedicine
- Albumin Nanoparticle Development for Nanomedicine
- Lipid Nanoparticle Development for Nanomedicine
- Polymeric Nanoparticle Development for Nanomedicine
- Biomimetic Nanoparticle Development for Nanomedicine
- Environmentally Responsive Nanoparticle Development for Nanomedicine
- Nanosuspension Development for Nanomedicine
- Liposome Development for Nanomedicine
- Exosome Development for Nanomedicine
- Nanostructured Lipid Carrier Development for Nanomedicine
- Nanoemulsion Development for Nanomedicine
- Nanocrystal Development for Nanomedicine
- Polymeric Micelle Development for Nanomedicine
- Nanofiber Development for Nanomedicine
- Nanocapsule Development for Nanomedicine
- Nanogel Development for Nanomedicine
- Microparticle Development for Nanomedicine
- Targeting Nanoformulation Development
- Generic Nanoformulation Development
- Oral Nanoformulation Development
- Nanoparticle Development for Nanomedicine
- Nanoformulation Analysis
- Characterization of APIs and Nanocarriers
- Nanoformulation Mean Particle Size Distribution and Testing Service
- Zeta Potential Measurement Service for Nanoformulations
- Nanostructure Characteristics Analytical Service
- Crystallinity Testing Service for Nanoformulations
- Microscopic Morphology Testing of Nanoparticles
- Nanoformulation Surface Property Testing Service
- Nanoformulation Molecular Weight Distribution Testing Service
- Nanoformulation Encapsulation Efficiency Testing Service
- Nanoformulation Drug Loading Testing Service
- Nanoparticle Concentration Testing Service
- Drug Release Testing Service for Nanocarriers
- Nanoformulation Thermal Performance Analysis
- Nanoformulation Critical Micelle Concentration Testing Service
- Nanoformulation Critical Aggregation Concentration Testing Service
- Nanoformulation Elemental Impurity Testing Service
- Nanoformulation Stability Testing Service
- Nanoformulation Residual Solvent Testing Service
- In Vitro Release Determination of Nanoformulations
- Endotoxin and Sterility Testing Service of Injectable Nanoformulations
- Nanoformulation Analytical Method Development and Optimization
- Nanoformulation Analytical Method Validation and Transfer
- Nanoformulation Biological Evaluation
- Nanoformulation Batch Release Testing Services
- Nanoformulation Pilot Scale-up Services
- Nanoformulation Development
- Technologies
- Nanotechnology Platforms for Nanoformulations
- Nanotechnology for Drug Delivery
- Targeting Drug Delivery System for Nanoformulations
- Microparticle Drug Delivery System for Nanoformulations
- Colloidal Drug Delivery System for Nanoformulations
- Self-emulsifying Drug Delivery System for Nanoformulations
- Exosome Drug Delivery System for Nanoformulations
- In Vivo Drug Delivery Strategies Based on Nanotechnology
- Co-encapsulation Nanomaterial Drug Delivery System for Nanoformulations
- Selective Organ Targeting Nanoparticle Platform for Nanoformulations
- Nanoformulation Technology for Biologics
- Nanochip Technology for Nanoformulations
- Applications
- Order Online
- Company
- Inquiry

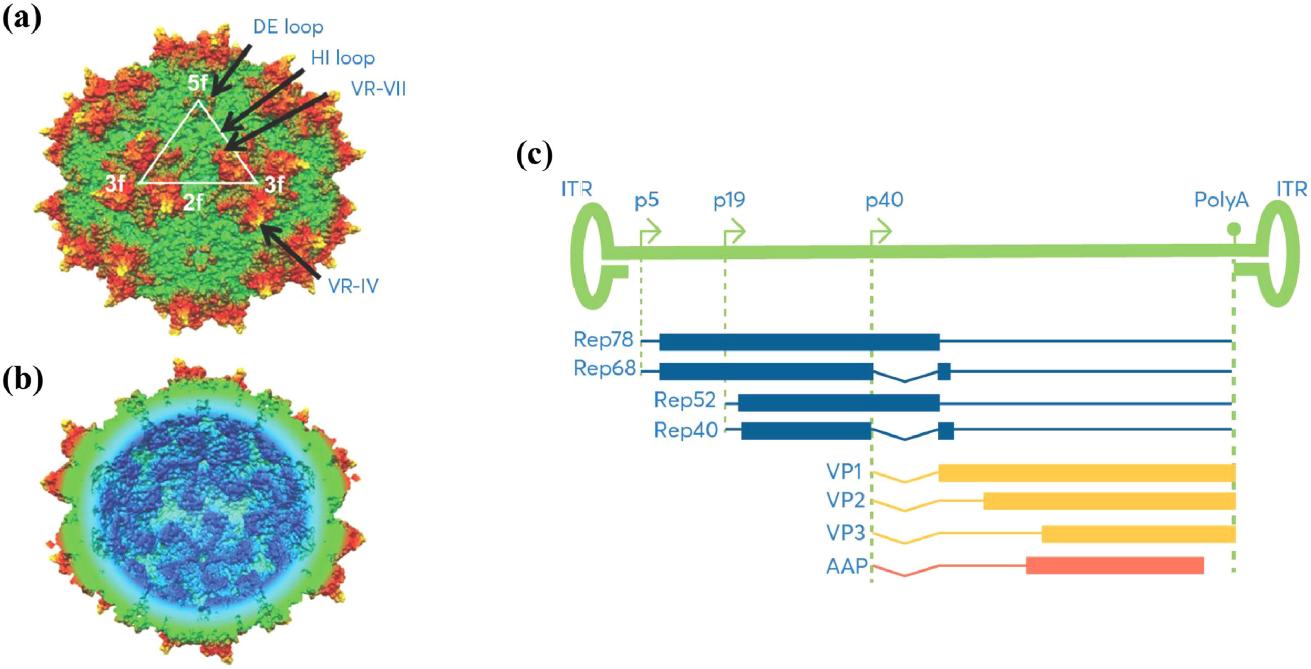 Fig.1 Schematic representation of AAV genomic structure. (Arvind Srivastava, et al. 2021)
Fig.1 Schematic representation of AAV genomic structure. (Arvind Srivastava, et al. 2021) 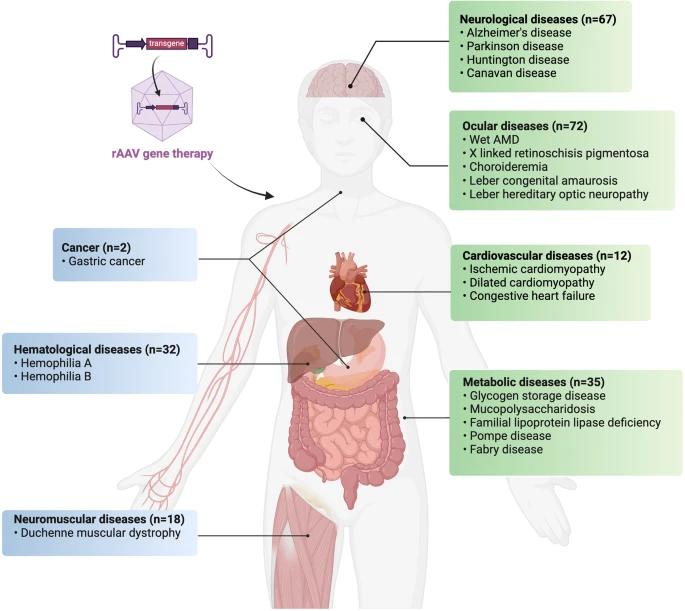 Fig.2 Current clinical applications of rAAV in major human diseases. (Jiang-Hui Wang, et al. 2024)
Fig.2 Current clinical applications of rAAV in major human diseases. (Jiang-Hui Wang, et al. 2024) 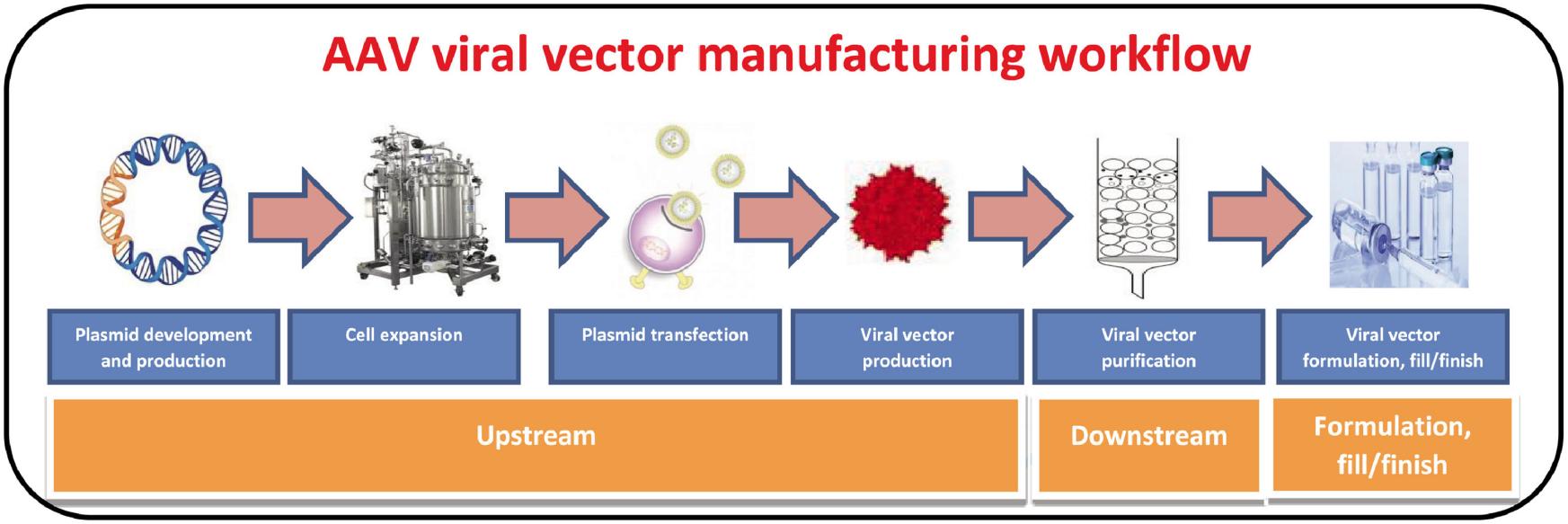 Fig.3 Viral vector manufacturing process workflow, which involves various upstream, downstream, formulation, and fill/finish steps. (Arvind Srivastava, et al. 2021)
Fig.3 Viral vector manufacturing process workflow, which involves various upstream, downstream, formulation, and fill/finish steps. (Arvind Srivastava, et al. 2021) 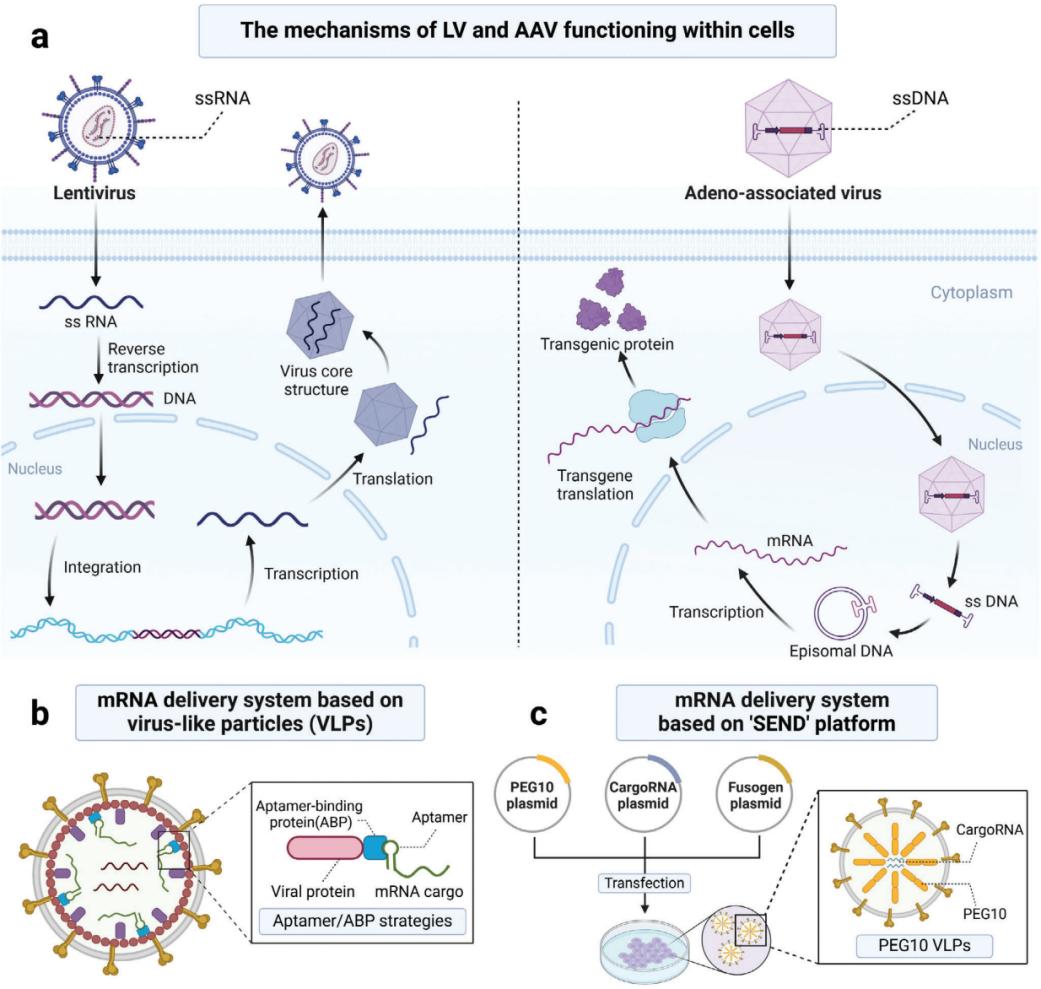 Fig.4 Virus-derived mRNA delivery system. (Menghao Yin, et al. 2024)
Fig.4 Virus-derived mRNA delivery system. (Menghao Yin, et al. 2024) 
