Transdermal Liposome Formulation Development
Inquiry
At CD Formulation, the research and development center for transdermal liposome products has a long-standing establishment, and our products have consistently demonstrated exceptional transdermal efficacy. Furthermore, throughout the years, beside conventional liposomes, our team of scientists also has successfully overcome numerous challenges in the development of deformable liposomes including ethosomes (such as propylene glycol liposomes), transethosomes, and niosomes. We provide our customers with professional and verifiable solutions while delivering outstanding transdermal liposome products that meet their satisfaction. Most importantly, our proficient team will tailor a range of exclusive services to systematically address your project concerns: from pre-formulation development to in vitro evaluation of product transdermality or from pilot stage advancement to scale-up resolution for an array of issues.
Why Develop Transdermal Liposome Formulations?
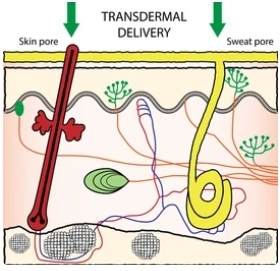
Transdermal systems have emerged as a promising, widely accepted, and popular novel approach for drug delivery. These systems offer numerous advantages, including easy administration, simplicity of operation, minimal systemic exposure, reduced discomfort, enhanced flexibility and adjustability, and controlled release capabilities leading to prolonged therapeutic effects. However, transdermal liposomes still encounter several challenges with poor skin permeability being the primary obstacle. Traditional liposomes fail to traverse the intact stratum corneum and instead decompose or fuse at the skin surface. Therefore, there is an emerging imperative to enhance the membrane materials employed in liposomes through the development of "soft" liposomes comprised of lipids possessing exceedingly low transition temperatures that render them inherently more fluidic in nature. Liposomal formulations designed for transdermal absorption typically encompass transfersomes, ethosomes, precursor liposomes, terpesomes as well as liposome-like vesicles which possess superior ability to penetrate the stratum corneum barrier with relative ease. Notably, they can serve as carriers facilitating improved retention of active substances within the skin and subcutaneous tissues.
Explore Our Transdermal Liposome Formulation Development Services
As a company with cutting-edge technology, our services mainly focus on the development of transdermal liposome formulations, which include the full range of services provided by CD Formulation. Our service team possesses extensive industry experience and profound expertise, enabling us to deliver tailor-made service solutions that cater to diverse customer needs. Furthermore, we are dedicated to conducting research and development on novel transdermal liposome formulations to meet the evolving demands of both the market and our esteemed clientele. In essence, we offer comprehensive services encompassing every stage of transdermal liposome formulation development, establishing ourselves as your reliable partner.
Our services include but are not limited to:
Feasibility analysis and consulting services
The feasibility study of your project can be conducted by our team prior to the official project launch, based on the comprehensive information obtained through effective communication with you.
Pre-formulation and formulation design
The service entails conducting pre-formulation and formula design based on product objectives, followed by the selection of various APIs, excipients, preparation methods, manufacturing routes, etc.
Process optimization
Optimizing various parameters of the formula product through characterization analysis, such as assessing transdermal absorption performance via in vitro transdermal permeation experiments, to provide guidance for liposome formula development.
Quality system establishment and control
The QbD approach necessitates the establishment of a robust quality analytical system that can be verified throughout the entire life cycle.
Our Workflow of Transdermal Liposome Formulation Development
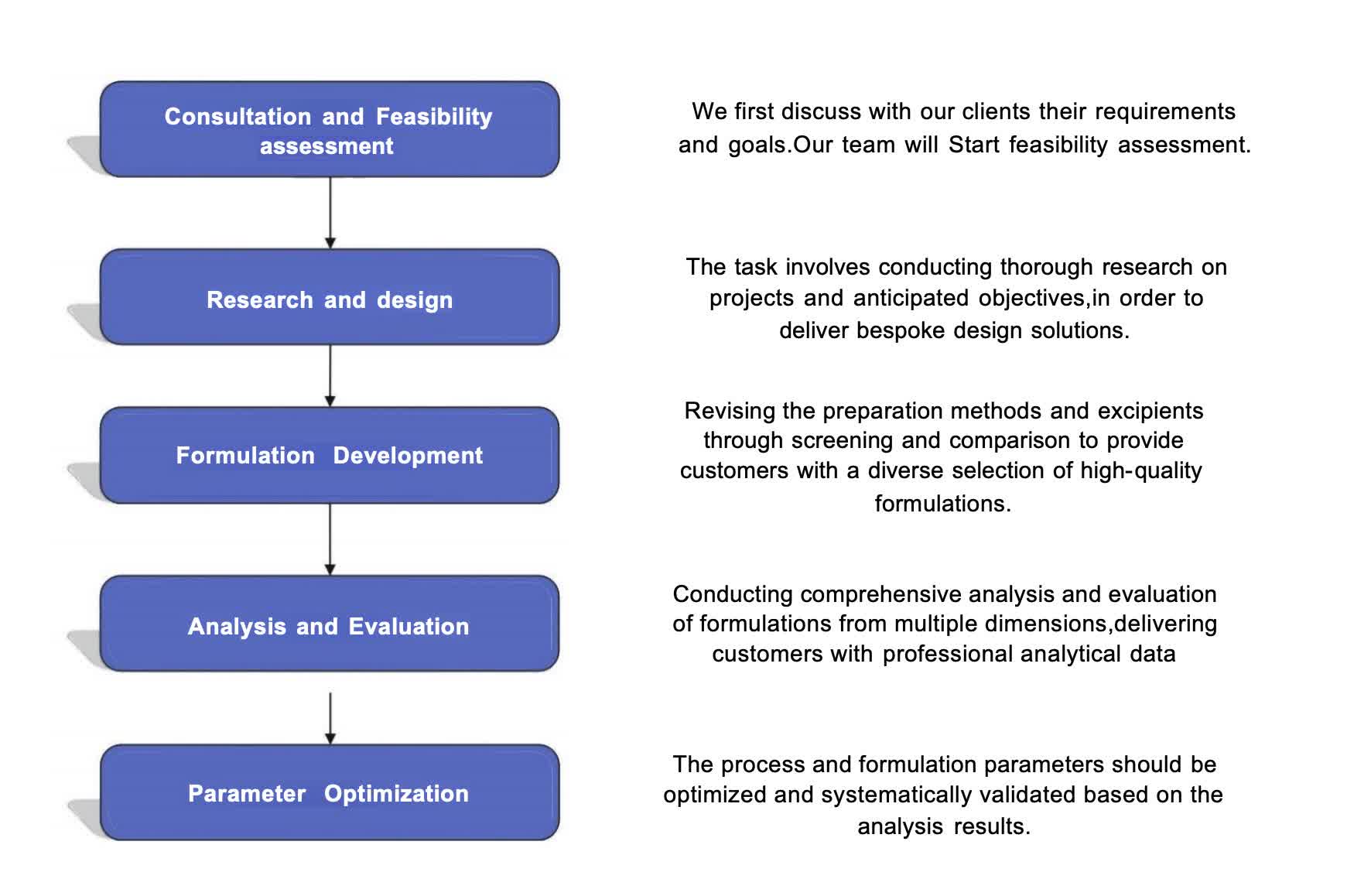 Fig.1 The workflow of transdermal liposome formulation development (CD Formulation).
Fig.1 The workflow of transdermal liposome formulation development (CD Formulation).
Our Platforms for Transdermal Liposome Formulation Development
| Platform |
Contents |
| Formulation Design Platform |
Preformulation, formulation feasibility, and prototype development; screening of edge activators, oil phase, aqueous phase, pH of aqueous phase, relevant ion concentration, drug-to-lipid ratio, etc. |
| Process Development Platform |
Parameters optimization from laboratory, pilot to production scales. Comparison and screening of various preparation methods. |
| Percutaneous Permeation Assessment Platform |
Several in vitro, ex vivo, and in vivo models have been established to analyze the percutaneous absorption. In vivo models; Cell-based models; multi-layer synthetic membrane engineered to mimic human skin, etc. |
| Analysis and Characterization Platform |
ICP(inductively coupled plasma spectroscopy); LC-MS/MS(liquid chromatography tandem mass spectrometry), HPLC(high-performance liquid chromatography), IR (fourier infrared spectroscopy), (nuclear magnetic resonance spectroscopy)NMR, SEM(scanning electron microscope), TEM(transmission electron microscopy), DLS(dynamic laser light scattering), MALDI-TOF MS(matrix-assisted laser desorption ionization time-of-flight mass spectrometry), FT-ICRMS (fourier transform ion cyclotron resonance mass spectrometer), Orbitrap MS, IMS-Q-TOF MS, etc. |
Our Expertise for Transdermal Liposome Formulation Development
- Design and screening of pre-formulation and formulation: determination of solvents and co-solvents, various excipient screening, pH testing, qualitative and quantitative ingredient selection and optimization, process development, and analytical method development and validation.
- Characterizations: physicochemical studies encompass encapsulation rate, drug loading, zeta potential, particle size distribution, deformability index, distribution analysis of active substances within liposomes; studies on degradation products of both lipids and active substances, viscosity measurements, lipid phase transition temperature determination, etc.
- In vitro study and stability evaluations: conventional and systematic stability according to ICH. Transdermal studies based on various in vitro models.
- Manufacturing process development: Scale up from lab to pilot to commercialization, including studying packaging material compatibility and evaluating various sterilization technologies and their impact on the product.
Applications of Transdermal Liposome Formulations
- Cosmetics. Transdermal liposome technology improves the bioavailability of active ingredients and enables personalized care for different skin types.
- Medical Science. Transdermal liposome technology in medical science has important application value, as it allows drugs to enter the skin and avoid side effects of oral administration, while improving efficacy through targeted drug release at the lesion site and reducing impact on normal tissues for safer treatment.
Published Data
Technology: deformable liposome gel technology
Journal: International Journal of Nanomedicine
IF: 8.1
Published: 2020
Results: In this study, Meloxicam liposomes, transferrin liposomes, and flavonoid liposomes were developed. The findings demonstrated that the vesicle size of the deformable liposome gel was below 120 nm, with higher encapsulation efficiency compared to conventional liposome gel and non-liposome gel. The prepared deformable liposome gel exhibited excellent permeability and in vitro efficacy.
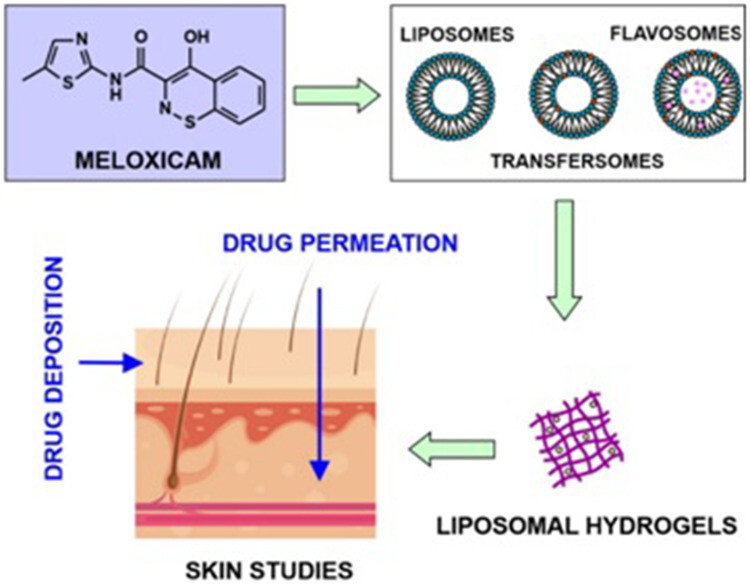 Fig.2 Flow diagram of liposomal hydrogel formulation preparation. (Zhang, Z. J., et al., 2020)
Fig.2 Flow diagram of liposomal hydrogel formulation preparation. (Zhang, Z. J., et al., 2020)
Our esteemed scientists at CD Formulations are thrilled to address any inquiries you may have regarding the groundbreaking development of transdermal liposomes. We also provide unparalleled consultation services to advance your projects. If you're interested in availing our exceptional services or require further correspondence, please feel free to contact us.
References
- Phatale V, Vaiphei KK, et al. Overcoming skin barriers through advanced transdermal drug delivery approaches. J Control Release. 2022, 351: 361-380.
- Schlich M, Musazzi UM, et al. Design and development of topical liposomal formulations in a regulatory perspective. Drug Deliv Transl Res. 2022,12(8): 1811-1828.
- Palac, Z., Engesland, A., et al. Liposomes for (trans)dermal drug delivery: the skin-PVPA as a novel in vitro stratum corneum model in formulation development. Journal of Liposome Research, 2014, 24(4): 313–322.
- Elmoslemany RM, Abdallah OY, et al. Propylene glycol liposomes as a topical delivery system for miconazole nitrate: comparison with conventional liposomes. AAPS PharmSciTech. 2012 Jun,13(2): 723-31.
- Zhang, Z. J., et al. Deformable Liposomal Hydrogel for Dermal and Transdermal Delivery of Meloxicam. International Journal of Nanomedicine, 2020, 15: 9319–9335.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services





