Oral Liposome Formulation Development
Inquiry
At CD Formulation, our oral liposome research center is dedicated to assisting clients in overcoming various challenges in the development of oral liposomes. We have decades of experience in developing oral liposome projects, particularly focusing on maintaining liposome integrity, prolonging gastrointestinal residence time, and enhancing liposome stability. At present, our oral liposomes primarily target unstable molecules, such as proteins and many precursor drugs. Regardless of your goals of enhancing drug bioavailability, reducing drug side effects, enhancing drug stability, or addressing large-scale production challenges, we can offer the optimal solution for you. We aim to provide our customers with high-quality and efficient oral liposome formulations to meet the needs of various industries and application scenarios.
Why Develop Oral Liposome Formulations?
Liposomes exhibit promising potential as carriers for drugs and nutrients due to their high encapsulation capacity, biocompatibility, and safety profile. However, challenges in oral delivery arise from issues such as instability within the gastrointestinal tract, limited biofilm penetration ability, and difficulties in large-scale production. Strategies including adjusting formulation composition and implementing surface coating techniques have been explored to enhance liposome stability. By optimizing the lipid bilayer composition, drug solubility can be improved while maintaining optimal concentration levels in vivo. This facilitates absorption while minimizing interference from food intake and individual variations. Formulation development plays a crucial role in advancing the field of oral liposomes.
Our Oral Liposome Formulation Development Services
To address major challenges in the formulation of oral liposomes, we can provide you with the following services to improve the stability and absorption of liposomes to improve the efficiency of oral delivery of liposomes.
Formulation Optimization
The services we offer encompass various phases of oral lipid formulation development, ranging from laboratory-stage formula optimization to small-scale, pilot, and scale-up stages.
Phospholipid Functional Modification
In addition to traditional formulation development services, we also provide you with phospholipid functional modification services to improve the stability of liposomes. At the same time, we can also match your phospholipid system with different phase transition temperatures to meet your project needs.
Polymer Coatings Services
Based on our many years of research and development experience in the field of oral liposome formulation development, polymer coatings can protect the payload to varying extents, mainly by improving the adhesion to enhance the permeability of liposomes. We can offer polymer coatings services for liposomes including long-chain polyethylene glycol, chitosan, polysaccharide, protein, and its derivatives.
Ligand-mediated Services
We can provide ligand-mediated services that can also improve intestinal targeting, thereby improving the stability and bioavailability of oral liposomes.
Our Workflow of Oral Liposome Formulation Development
The formulation of liposomes is determined by the active ingredient, preparation method, type of phospholipid used, and intended application. The services provided by CD Formulation for oral liposome formulation development are primarily categorized into the following aspects:
- Formulation design: including screening of oil phase, aqueous phase, pH of aqueous phase, relevant ion concentration, drug-to-lipid ratio, etc.
- Process development: comparing preparation methods and optimizing process parameters.
- Liposome characterization: encapsulation efficiency, drug-loading rate, morphology and number of membrane layers, surface potential, particle size distribution, and stability analysis, etc.
Our Platforms for Oral Liposome Formulation Development
| Formulation Design Platform |
Preformulation, formulation feasibility, and prototype development; screening of oil phase, aqueous phase, pH of aqueous phase, relevant ion concentration, drug-to-lipid ratio, etc. |
| Process Development Platform |
Our scientists can develop oral liposomes at laboratory, pilot, and production scales while optimizing process parameters at the early development stage to scale up. We also compare preparation methods to optimize process parameters. |
| Analysis and Characterization Platform |
We offer multiple analysis methods to confirm the stability of oral liposomes including encapsulation efficiency, drug-loading rate, morphology and number of membrane layers, surface potential, particle size distribution, stability analysis, etc. Our analytical laboratory center is equipped with a variety of advanced equipment. |
Our Advantages in Oral Liposome Formulation Development
- Advanced Technology. Unique platform and professional equipment.
- Expertise. Proven research portfolio solutions and expert teams in oral liposome development.
- Rigorous. The verifiable development methods are developed in accordance with the guidelines provided by ICH and IQ.
- Flexibility. The project design is collaboratively and customarily tailored, ensuring successful execution.
Applications of Oral Liposome Formulation
- Food and nutritional supplements: Liposomes enhance the stability, bioavailability, and targeted delivery of active ingredients in food and health products to augment their nutritional value, improve sensory attributes, and prolong shelf life.
- Pharmaceutical field: Liposomes are utilized for the development of unstable biomacromolecules or prodrugs in specific therapeutic areas such as cancer, gastrointestinal diseases, diabetes, cardiovascular diseases, and neurodegenerative diseases.
- Vaccines: Liposomes can serve as oral delivery vectors to elicit mucosal immune responses.
Published Data
Technology: a new method to prepare and compound child-friendly oral formulations based on liposomal multilamellar vesicle (MLV) technology
Journal: International Journal of Pharmaceutics
IF: 5.8
Published: 2022
Results: In this research, the author developed the MLV platform technology for making child-friendly oral liquid formulations of weak base drugs to support the compounding of adult solid dosage forms into pediatric formulations.
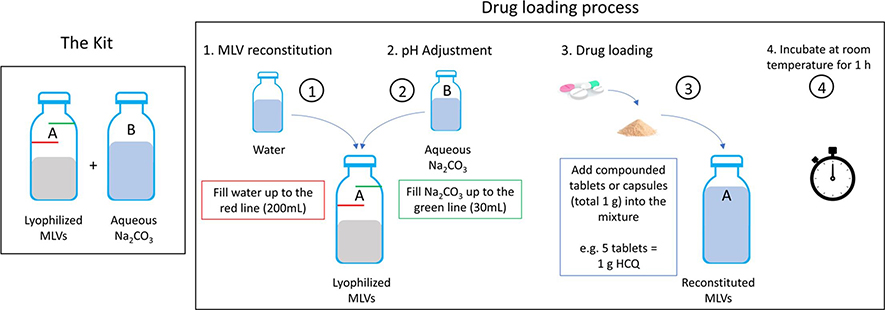 Fig.1 The drug loading process. (Al Fayez N, et al., 2022)
Fig.1 The drug loading process. (Al Fayez N, et al., 2022)
Our scientists at CD Formulation are delighted to answer any questions you may have regarding the development of oral liposomes and provide consultation services for the development of your projects. If you are interested in our services or would like further communication, please feel free to contact us.
References
- He H, Lu Y, et al. Adapting liposomes for oral drug delivery. Acta Pharm Sin B. 2019, 9(1): 36-48.
- Al Fayez N, Böttger R, et al. Development of a child-friendly oral drug formulation using liposomal multilamellar vesicle technology. International Journal of Pharmaceutics. 2022, Sep; 625: 122107.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services




