Novel Technologies for Liposome Preparation
Inquiry
At CD Formulation, we specialize in innovative liposome preparation technologies, enhancing the efficiency of drug delivery systems. With our expertise, we support the development of highly efficient liposome products using state-of-the-art techniques. Our dedicated technology center assists researchers in overcoming formulation challenges to meet diverse research needs.
Challenges in Traditional Liposome Preparation
Despite the widespread use and convenience of traditional methods for producing liposome nanoparticles, numerous challenges persist. These include the substantial requirement for organic solvents, which pose environmental and human health risks, as well as the necessity for multiple homogenization steps that demand significant energy input and are unsuitable for large-scale production. In response to stability and formulation concerns associated with these methods, innovative liposome technologies have been developed, giving rise to different new approaches.
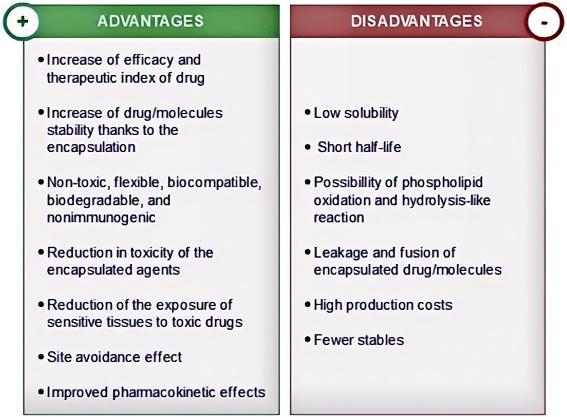 Fig.1 Advantages and disadvantages of traditional liposomal applications. (Maja L, et al, 2020)
Fig.1 Advantages and disadvantages of traditional liposomal applications. (Maja L, et al, 2020)
Innovative Liposome Preparation Techniques
Currently, novel technologies are being employed to complement traditional methods in industrial production. These include supercritical reverse phase evaporation (SCRPE), freeze drying, various heating techniques, spray drying, and enhanced ethanol injection methods that are gaining increasing attention.
In response to the challenges of toxicity and biodegradability associated with traditional methods, a variety of novel technologies have emerged. Represented around supercritical fluid technology (SCF), it stands out as a prominent green production method that has garnered significant attention and offers a widely embraced alternative to conventional lipid production techniques. The alternatives to conventional liposome production techniques, include the supercritical antisolvent method (SAS), supercritical reverse phase evaporation (SCRPE), expanded liquid organic solvent suspension under reduced pressure (DELOS), and supercritical assisted liposome formation (superlipids).
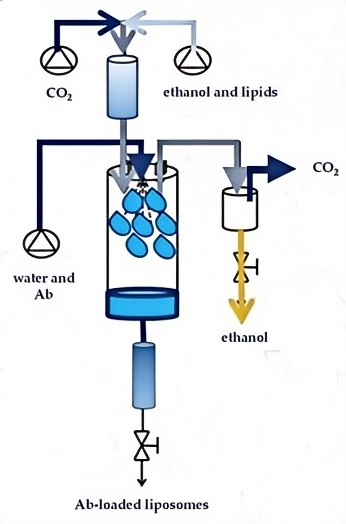 Fig.2 A supercritical fluid-assisted process for liposomes. (Carugo, D., et al, 2016)
Fig.2 A supercritical fluid-assisted process for liposomes. (Carugo, D., et al, 2016)
Our Novel Technologies for Liposome Preparation
Freeze-drying consists of two steps: firstly, the sample is frozen at an appropriate temperature and then dried at room temperature. This results in liposomes with an average size of 100-300 nm after hydration. The process necessitates the selection of suitable freeze-drying protectants.
Supercritical fluids (SCF) exhibit a combination of ideal properties from both liquids and gases. As a result, SCF is increasingly being utilized as a replacement for organic solvents due to its ability to achieve more efficient separation and purification. We tailor liposome products to align with our customer's specific requirements through the integration of this cutting-edge technology.
In this approach, lipids are typically dissolved in a transfer medium, which is subsequently removed. Lipid particles undergo spontaneous self-assembly in the bulk phase during the process of hydrating the membrane or removing the transfer medium (such as an organic solvent or detergent solution).
The method is a relatively recent technique that has emerged in the past few years and is commonly employed for emulsion preparation. Based on our experience, the size of lipid nanoparticles is directly proportional to the amount of lipids used in the method. Moreover, an increase in lipid content leads to a reduction in the flow rate of the lipid phase and lower membrane permeability. The size of lipid nanoparticles is primarily influenced by factors such as membrane surface area, reaction time, lipid flow rate, and type and concentration of emulsifier.
State-of-the-Art Platforms for Liposome Preparation
| Facilities and Techniques |
Specifics |
Freeze-Drying Techniques and Facilities
 |
- Liposome lyophilization involves three critical processes: pre-freezing, sublimation drying, and reconstitution drying.
- This advanced technology can be applied to produce both traditional liposomal drugs and innovative nanoliposomal drugs, enhancing drug targeting and therapeutic effects.
- Our lyophilizers can meet the requirements of research, development, and production.
|
Supercritical Fluid-Assisted Techniques and Facilities
 |
- Supercritical extraction equipment is widely employed in the pharmaceutical, food, and environmental sectors due to its high efficiency, environmentally friendly nature, and strong selectivity. It provides significant support for the progress and innovation of related industries.
- The primary equipment for supercritical extraction processes includes an extraction vessel, separation vessel, compressor, and heat exchanger; these components can be integrated into three typical process flows.
|
Microfluidic Techniques and Platform
 |
- Microfluidic techniques can effectively produce liposomes of controlled size by adjusting the flow rate ratios (FRR) of buffer to lipid and optimizing the FRR can enhance the performance of liposomes in terms of drug concentration, encapsulation efficiency, and stability.
- Different microfluidic techniques can be used to prepare liposomes, such as droplets, microfluidic dynamic focusing (MHF), pulsed jet flow, and similmicrofluidic.
|
Membrane Contactor Techniques and Facilities
 |
- The hollow fiber tubes coated with nanoporous membranes serve as the contact area for the aqueous/oil phase, and the organic solution containing lipids passes through the hollow fiber membrane contactor.
- The feed liquid flows in a gentle laminar state, which can effectively reduce the shear stress on the feed liquid containing the target product, thereby favorably maintaining the structural and functional integrity of the product, which is particularly important for liposome products that are sensitive to shear.
- Our platform supports the development and production of hollow fiber membrane contactors based on different specifications.
|
Advantages of Our Novel Liposome Techniques
- Novel techniques for formulating and optimizing parameters. We possess expertise in novel liposome preparation processes, encompassing the selection of methods, formulation design, process optimization, and validation analysis. The novel techniques include but are not limited to the freeze-drying technique, supercritical fluid-assisted technique, microfluidic technique, and membrane contactor technique.
- Precision equipment for research and development. Our platform is equipped with state-of-the-art professional tools suitable for a wide range of technical applications, catering to the specific requirements at each stage of liposome research and production. These tools encompass high-efficiency centrifuges, precision analytical instruments, automated production lines, and more, providing comprehensive support across diverse fields. During the liposome research and development phase, we offer tailored design and experimental validation based on customer needs; in the production phase, we ensure product stability and reliability to meet stringent market demands. Whether in the pharmaceuticals, food, or cosmetics industries, we deliver optimal solutions while meticulously considering every detail.
- Highly skilled core team. The core team comprises individuals from renowned biopharmaceutical companies and prestigious universities, each possessing extensive expertise in chemistry, materials science, biotechnology, medicine, and liposome technology. They bring substantial experience in liposome research, quality management, and industrial production.
Explore Our Liposome Preparation Services
Liposome Formulation Development
We possess unique liposome formulation technology, which can produce various types of liposomes, including single-chamber liposomes, multi-chamber liposomes and nano-liposomes. Our team of experts provides comprehensive services for the development of liposome formulations.
Liposome Analysis and Characterization
The study of liposome quality characterization and critical quality attributes (CQAs) can be utilized for formulation process screening and stability testing, as well as serving as a reference and foundation for subsequent non-clinical research.
As a leader in nanoparticle research, CD Formulation is dedicated to providing innovative and customized liposome solutions for researchers. We drive progress in liposome technology across various industries. Please feel free to contact us if you need any assistance.
References
-
Maja L, Željko K, et al. Sustainable technologies for liposome preparation. The Journal of Supercritical Fluids. 2020. Nov 1; 165: 104984.
Ferrari PF, Trucillo P, et al. Operating Parameters Optimization for the Production of Liposomes Loaded with Antibodies Using a Supercritical Fluid-Assisted Process. Processes. 2023; 11(3): 663.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 Advantages and disadvantages of traditional liposomal applications. (Maja L, et al, 2020)
Fig.1 Advantages and disadvantages of traditional liposomal applications. (Maja L, et al, 2020) Fig.2 A supercritical fluid-assisted process for liposomes. (Carugo, D., et al, 2016)
Fig.2 A supercritical fluid-assisted process for liposomes. (Carugo, D., et al, 2016)




