Liposome Vaccine Encapsulation Service
Inquiry
Liposomes have emerged as a critical adjuvant carrier system in vaccinology, celebrated for their multifunctionality and superior biocompatibility. Over the past decades, researchers have dedicated significant efforts to developing liposome-based vaccines to enhance efficacy and stability. CD Formulation leverages cutting-edge liposome encapsulation technology to assist enterprises and institutions in creating stable and efficient vaccine-encapsulated liposomes. Our expertise ensures high-quality, tailored systems that meet the unique demands of advanced vaccinology research.
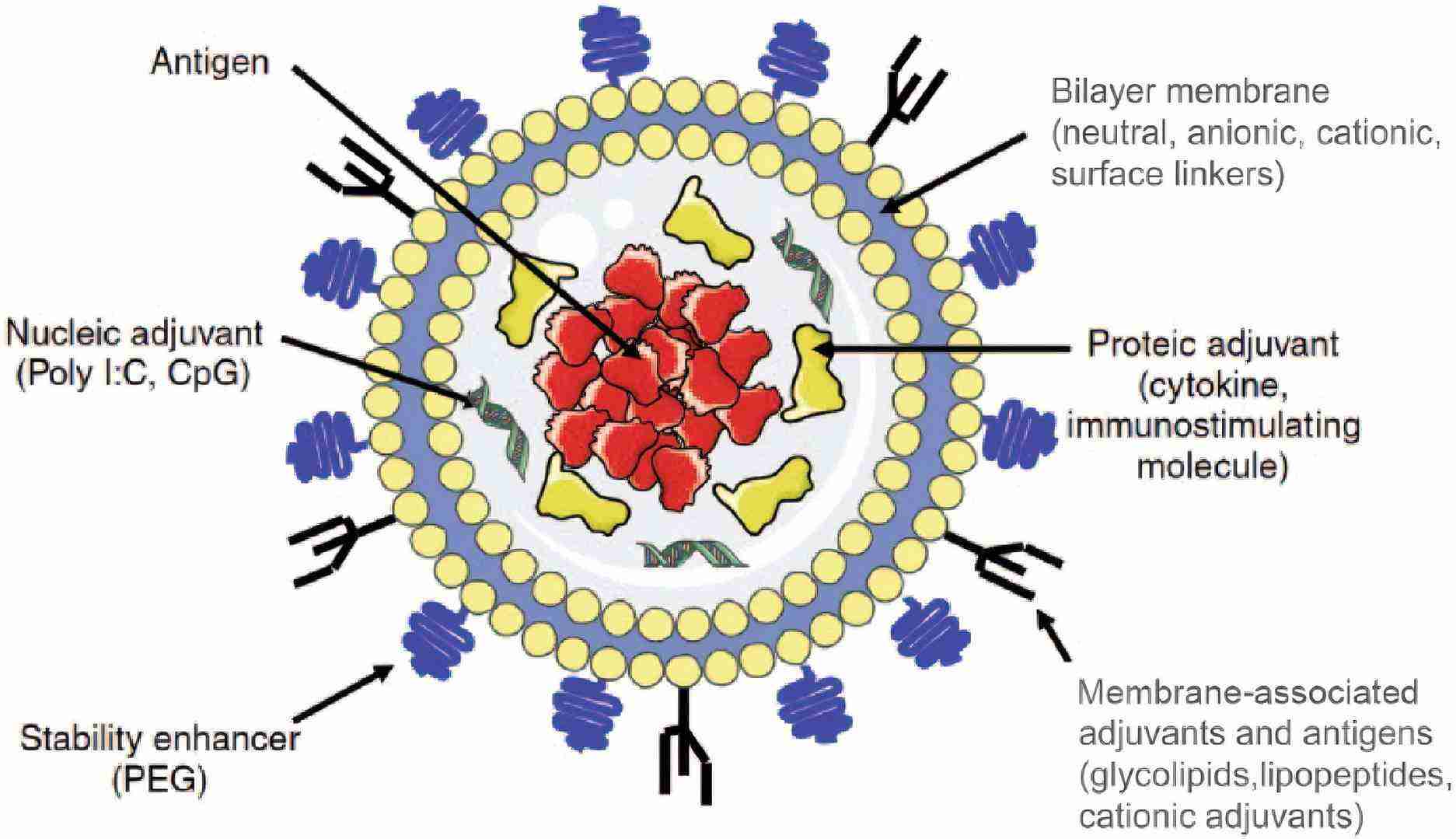 Fig.1 The illustration of liposomes as adjuvants and vaccine delivery systems. (Schwendener RA, et al, 2014)
Fig.1 The illustration of liposomes as adjuvants and vaccine delivery systems. (Schwendener RA, et al, 2014)
Importance of Liposome-Based Vaccine Encapsulation
Traditional vaccines do not require the use of adjuvants. They themselves contain antigens as well as additional components from bacteria or viruses that simultaneously effectively activate various elements of the innate immune system. Developing alternative strategies becomes more and more important as allergic and autoimmune reactions after vaccination, as well as the manufacturing and transporting challenges. Encapsulating antigens have these advantages:
- safeguarding antigens against premature degradation;
- enhancing uptake by antigen-presenting cells through passive or targeted transport;
- providing a reservoir for sustained antigen presentation;
- facilitating simultaneous delivery of antigens and adjuvants/immune stimulants to cells, thereby enabling modulation of the immune response type (humoral or cellular).
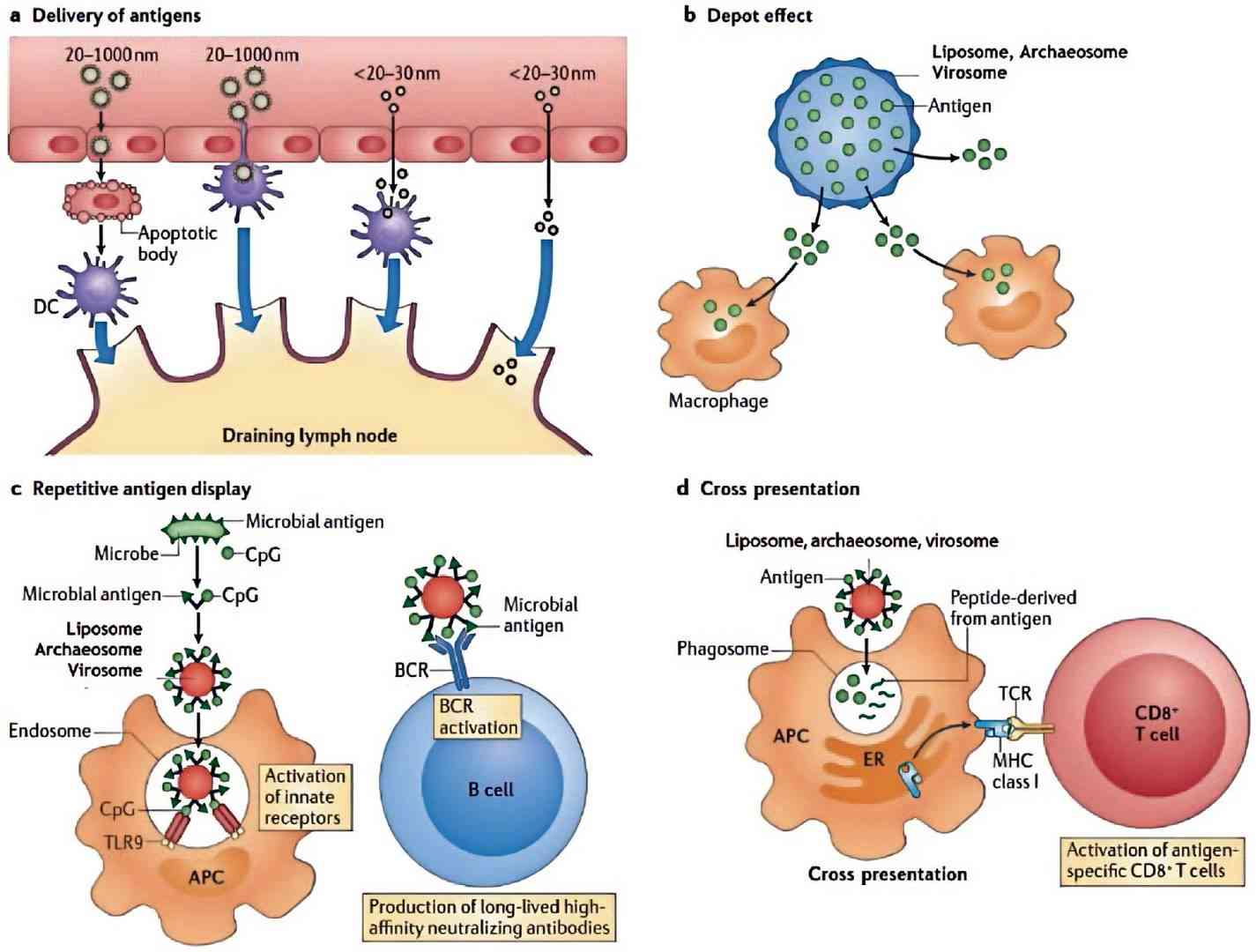 Fig.2 The mechanisms by which nanoparticles alter the induction of immune responses. (Schwendener RA, et al, 2014)
Fig.2 The mechanisms by which nanoparticles alter the induction of immune responses. (Schwendener RA, et al, 2014)
Overview of Our Liposome Vaccine Encapsulation Service
Antigen research services
Our research on vaccines includes viral vaccines, bacterial vaccines, genetically engineered vaccines, adjuvant vaccines, and innovative mRNA vaccines. Our liposome delivery service mainly focuses on genetically engineered vaccines and nucleic acid vaccines. We offer professional services for designing mRNA sequences and selecting nucleotide modifications for nucleic acid vaccines.
Formulation of vaccine-encapsulated liposomes
A significant advantage of liposomes as a vaccine delivery system is their inherent versatility and adaptability. By carefully screening the composition and preparation methods—including lipid composition, charge, size, electrostatic distribution, and the encapsulation or positioning of antigens or adjuvants—we can customize them to meet specific requirements. Water-soluble compounds (such as proteins, peptides, nucleic acids, carbohydrates, and semi-antigens) are generally encapsulated in the aqueous core, while lipophilic compounds (including lipid peptides, antigens, adjuvants, and linker molecules) are integrated into the lipid bilayer. Antigens can be affixed to the surface of liposomes through adsorption or stable chemical linkage.
Services for the development of vaccination pathways
Traditional vaccines are typically delivered via peripheral routes, and we remain dedicated to assisting our clients in exploring the potential of alternative administration routes. For instance, the contemporary approach of pulmonary vaccine delivery can be accomplished via nasal drops or by directly targeting the lung tissue.
Characterization service
To guarantee the efficacy of liposome vaccine design, we typically perform the following assessments: the expression levels of RNA coding, intracellular immunogenicity, the stability and toxicity associated with the chemical composition of the liposome vaccine, administration route, and therapeutic targets. Additionally, it is crucial to compare systemic administration of liposome vaccines with conventional intramuscular delivery methods.
Our Expertise in Liposome Vaccine Encapsulation
| Techniques and Platforms |
Specifics |
| Antigen Platform |
- Nucleotide modification and sequence design techniques.
- ORF sequence design.
- The strategic design and selection of sequences for gene vaccines and recombinant genes.
|
| Vaccine Encapsulation Technology |
- Development of liposome formulations.
- Stability assessments.
- Innovative liposome technologies, including ultra-deformable vesicles (transfersome), inter-lamellar cross-linked multilamellar vesicles, and solid-core liposomes.
|
| Characterization Platform |
- Apparent characterization. Structure morphology and shape of encapsulated drug.
- In vivo & in vitro evaluation. Such as release, leakage, and immunogenicity.
- Physical & chemistry evaluation. Including size distribution, potential analysis, and encapsulation efficiency.
|
Why Choose CD Formulation?
Exceptional Technological Expertise in Liposome Vaccine-Encapsulated
- At CD Formulation, this technology is suitable for different vaccines, including DNA, RNA, and recombinant genes, showing broad application potential.
- We are actively exploring a range of innovative vaccination strategies like nasal administration.
- We are expertise not only in preventive vaccines but also therapeutic vaccines.
An Outstanding and Powerful Evaluation System
- A range of sophisticated instruments for liposome vaccine characterization, covering the entire process.
- The stability research platform supports precisely evaluating store conditions and offers verifiable data.
- The quality research platform is specifically designed for liposome vaccines to efficiently evaluate the characteristics of related liposomes in vivo and in vitro.
- We develop exclusive human in vitro models to precisely assess the immunogenicity and safety profiles of vaccines.
An Exceptionally and Esteemed Professional Team
- We have effectively supported enterprises and academic institutions in the research and development of liposome vaccines.
- Our technical team is comprised of a diverse array of scientists from various disciplines.
Published Data
Technology: Liposome nanoparticles encapsulated with Yam Polysaccharide as vaccine adjuvants technique
Journal: Available at SSRN
IF: 2.32
Published: 2024
Results: The study introduced an innovative liposome immune adjuvant by incorporating Dioscorea polysaccharide into liposome nanoparticles using a customized microfluidic device, leveraging the synergistic immune-modulating effects of both components. A high-precision chip measuring 100 μm in height and 200 μm in width was designed with an embedded partitioned mixing channel to enhance fluid dynamics. Liposome nanoparticles were synthesized by optimizing the microfluidic chip's operational parameters. The formulation of YPL (Yam Polysaccharide-Loaded Liposome) was refined through response surface methodology, followed by characterization of its physical and chemical properties. In vitro cellular assays assessed YPL's ability to promote proliferation in mouse dendritic cells (DCs) and its influence on immune-related cytokine secretion, investigating its immunomodulatory effects. The sustained release profile indicated approximately 30 hours duration. YPL showed favorable biocompatibility while primarily stimulating dendritic cell proliferation, demonstrating significant immune enhancement potential as a novel vaccine adjuvant characterized by controlled particle size and high loading capacity for effective stimulation.
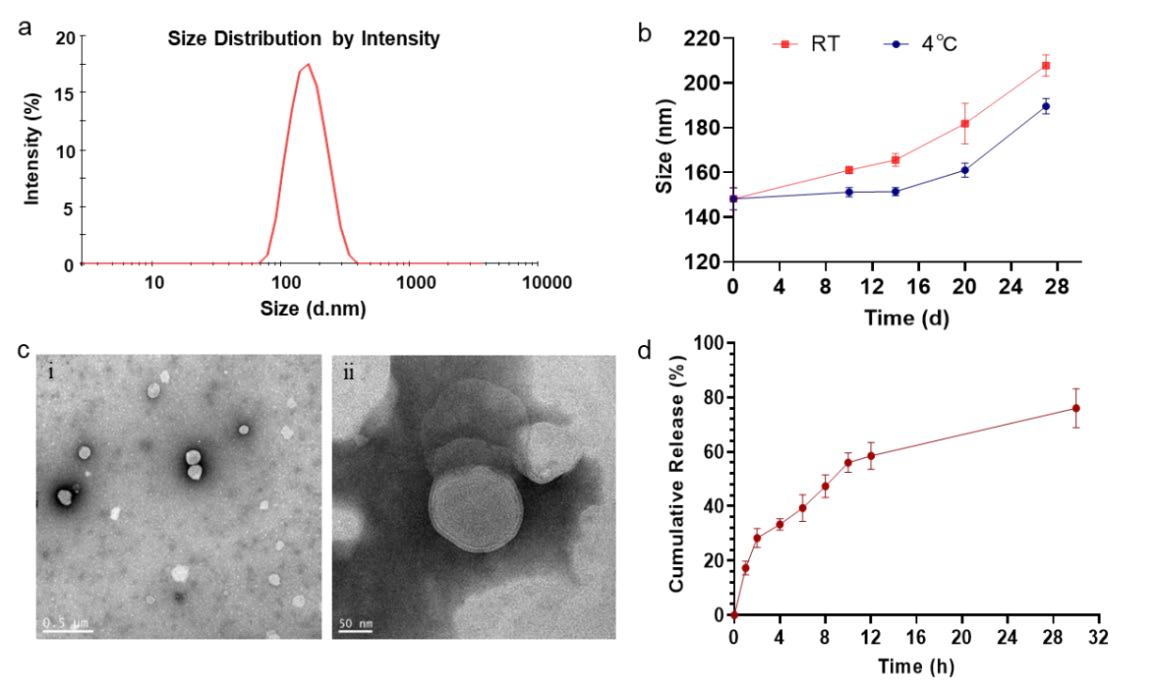 Fig. 3 Physical-chemical properties of YPL. (Serrano, A., et al, 2024)
Fig. 3 Physical-chemical properties of YPL. (Serrano, A., et al, 2024)
CD Formulation specializes in providing advanced liposome encapsulation technology for vaccine development, including mRNA, DNA, and recombinant protein vaccines. Our solutions are tailored to meet the specific needs of enterprises and research institutions, ensuring reliable and innovative support for vaccine R&D. Contact us to explore how we can contribute to your projects.
References
- Schwendener RA. Liposomes as vaccine delivery systems: a review of the recent advances. Therapeutic Advances in Vaccines. 2014; 2(6): 159-182
- Cui Y, Liu R, et al. Microfluidic Development of Liposome Nanoparticles Encapsulated with Yam Polysaccharide as Vaccine Adjuvants. Available at SSRN 4888708.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 The illustration of liposomes as adjuvants and vaccine delivery systems. (Schwendener RA, et al, 2014)
Fig.1 The illustration of liposomes as adjuvants and vaccine delivery systems. (Schwendener RA, et al, 2014) Fig.2 The mechanisms by which nanoparticles alter the induction of immune responses. (Schwendener RA, et al, 2014)
Fig.2 The mechanisms by which nanoparticles alter the induction of immune responses. (Schwendener RA, et al, 2014) Fig. 3 Physical-chemical properties of YPL. (Serrano, A., et al, 2024)
Fig. 3 Physical-chemical properties of YPL. (Serrano, A., et al, 2024)
