Liposome Surface Tension Study
Inquiry
From the point of view of the surface interface, through the use of the surface interface properties of drugs, excipients, medical materials, etc., the surface tension and contact Angle between the interface can be changed to achieve the purposes of wetting, solubilization, dispersion, and improving biocompatibility to meet the utilization requirements of liposome ophthalmic preparations. CD Formulation is equipped with an advanced technology platform for liposome surface tension study, which is committed to providing professional solutions for clients.
Why Conduct Liposome Surface Tension Study?
Wetting is the first step in the use and function of drugs in the body, such as drug dissolution. Surface tension is the main factor affecting drug wettability. The surface tension study focuses on adsorption kinetics, friction force, and surface interfacial tension. Eye drops are frequently employed to apply an active pharmaceutical ingredient (API) directly to the eye. The surface tension plays a key role in this process. The surface tension will affect the wetting and rupture time of the tear film, and the droplet volume is also related to the surface tension. Adding surface active ingredients is used to adjust the surface tension, as well as control and optimize the volume of eye drops. Therefore, the measurement of interfacial tension of ophthalmic liposomes is of great significance and is related to the clinical efficacy of ophthalmic liposomes. For example, the surface tension of drugs for dry eyes is higher than that of tears to increase the stability of tears and make the eyes more lubricated. However, the drug surface tension for pink eye is lower than normal tears to make the potion spread better in the eyes.
 Fig.1 The ramifications of surface energy in the form of surface tension. (Mozaffari Kosar, et al., 2018)
Fig.1 The ramifications of surface energy in the form of surface tension. (Mozaffari Kosar, et al., 2018)
Our Services for Liposome Surface Tension Study
As a transitional region between two phases, the surface interface also plays an important role in pharmaceutical research. The interfacial layer involved in pharmaceutical preparations is inseparable from the two-phase or three-phase interaction between gas, liquid, and solid. The interfacial interaction significantly impacts the final formulation of the preparation, as well as the dissolution and absorption of drugs within the human body. Therefore, our services include but are not limited to the following:
Surface Tension of Liposome Ophthalmic Preparations with Different Formulations
Pure liquid has only one molecule, so when the temperature and pressure are fixed, its surface tension is certain. The surface tension of the solution will change with the concentration. Different dosing concentrations may have a surface tension that does not pass. We provide surface tension testing services for liposome ophthalmic formulations in diverse concentrations. This service helps screen the effect of different formulations on surface tension.
Our Solutions for Liposomal Surface Tension
For different formulations, we provide customers with solutions to reduce or increase surface tension, facilitating the development of liposomes for eye use.
Our Platforms for Liposome Surface Tension Study
| Techniques |
Detailed Information |
| Statics measurement techniques |
- The measurement methods for statics are founded on the principle of force equilibrium. The determination of surface tension involves balancing it against other forces.
- The methods include the capillary rise method, Wilhelmy disk method, drop volume method, and maximum bubble pressure method.
|
| Dynamic detection technique |
- The kinetic measurement techniques vary depending on the specific method employed, such as the oscillating jet, spinning drop, and hanging drop methods.
|
Our Key Advantages in Liposome Surface Tension Study
- Advanced. The platform is equipped with professional facilities and technical platforms, including statics measurement techniques and dynamic detection techniques, which provide accurate and scientific analysis results.
- Scientific. To formulate and implement scientifically rigorous experimental protocols in strict accordance with industry standards.
- Cost-effective. We possess a wide array of testing instruments and analysis platforms to ensure the accuracy of your test results. Additionally, our project management follows a 1-to-1 service model to facilitate efficient and prompt communication.
Published Data
Technology: Interfacial tension technique in liposomal product physicochemical characterization
Journal: International Journal of Molecular Sciences.
IF: 5.6
Published: 2023
Results: Interfacial tension (IFT) measurements are being explored as a potential physicochemical characterization tool to aid in the characterization of liposomal products during their development and manufacturing. The interfacial tension of various Doxil® liposome suspension analogs in air and dodecane was measured using the hanging drop method of an optical tensiometer. The effects of liposome concentration, formulation (PEG and cholesterol content), the presence of encapsulated drugs, and average particle size were investigated. It was observed that Doxil®-like liposomes exhibit surfactant-like behaviors, with S-shaped interfacial tension and concentration curves. This behavior is largely influenced by the PEG content, and removing PEG from the formulation eliminates the surfactant-like behavior. The authors demonstrate for the first time that interfacial tension can detect certain changes in liposome formulation, such as PEG content, presence of encapsulated drugs, and size variations, making it a valuable complement to physicochemical characterization during liposome product development and manufacturing.
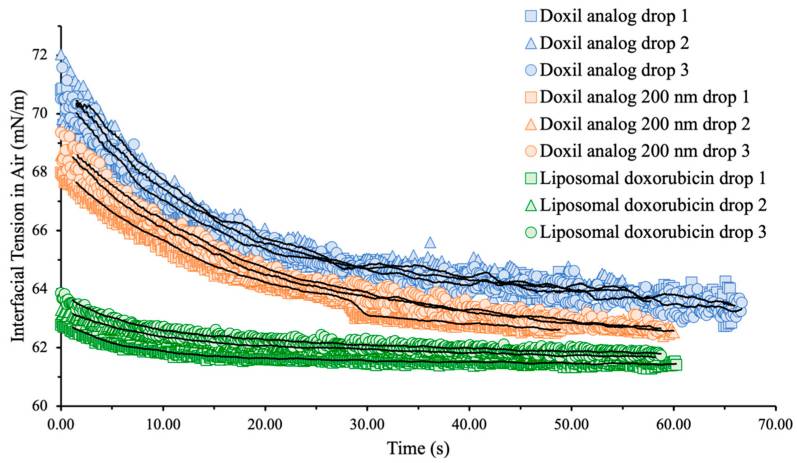 Fig.2 Interfacial tension evaluation of liposomes. (Mishra I, et al., 2023)
Fig.2 Interfacial tension evaluation of liposomes. (Mishra I, et al., 2023)
CD Formulation has developed a complete liposome analysis platform and process to provide customers with professional analysis solutions, especially in liposome surface tension studies. If you need any help, please contact us immediately.
References
- Mozaffari, Kosar; Yang, Shengyou, et al. Surface Energy and Nanoscale Mechanics. 2018. 10.1007.
- Mishra I, Garrett M, et al. Effect of Composition and Size on Surface Properties of Anti-Cancer Nanoparticles. Int J Mol Sci. 2023. Aug 30. 24(17): 13417.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 The ramifications of surface energy in the form of surface tension. (Mozaffari Kosar, et al., 2018)
Fig.1 The ramifications of surface energy in the form of surface tension. (Mozaffari Kosar, et al., 2018) Fig.2 Interfacial tension evaluation of liposomes. (Mishra I, et al., 2023)
Fig.2 Interfacial tension evaluation of liposomes. (Mishra I, et al., 2023)
