Liposome Stability and Storage Testing
Inquiry
Stability is a critical aspect of any drug delivery system, requiring careful consideration of all production factors. A liposome formulation must maintain its physical and chemical integrity throughout its shelf life while resisting external influences. Liposomes, being moderately unstable colloidal systems, demand precise attention to factors such as preparation techniques, lipid composition, storage conditions, and in-depth stability studies. CD Formulation offers specialized liposome stability and storage testing services to support the research and development efforts of pharmaceutical companies and research institutions. Our advanced methodologies ensure the reliability and longevity of liposome-based formulations, helping to optimize their quality and performance.
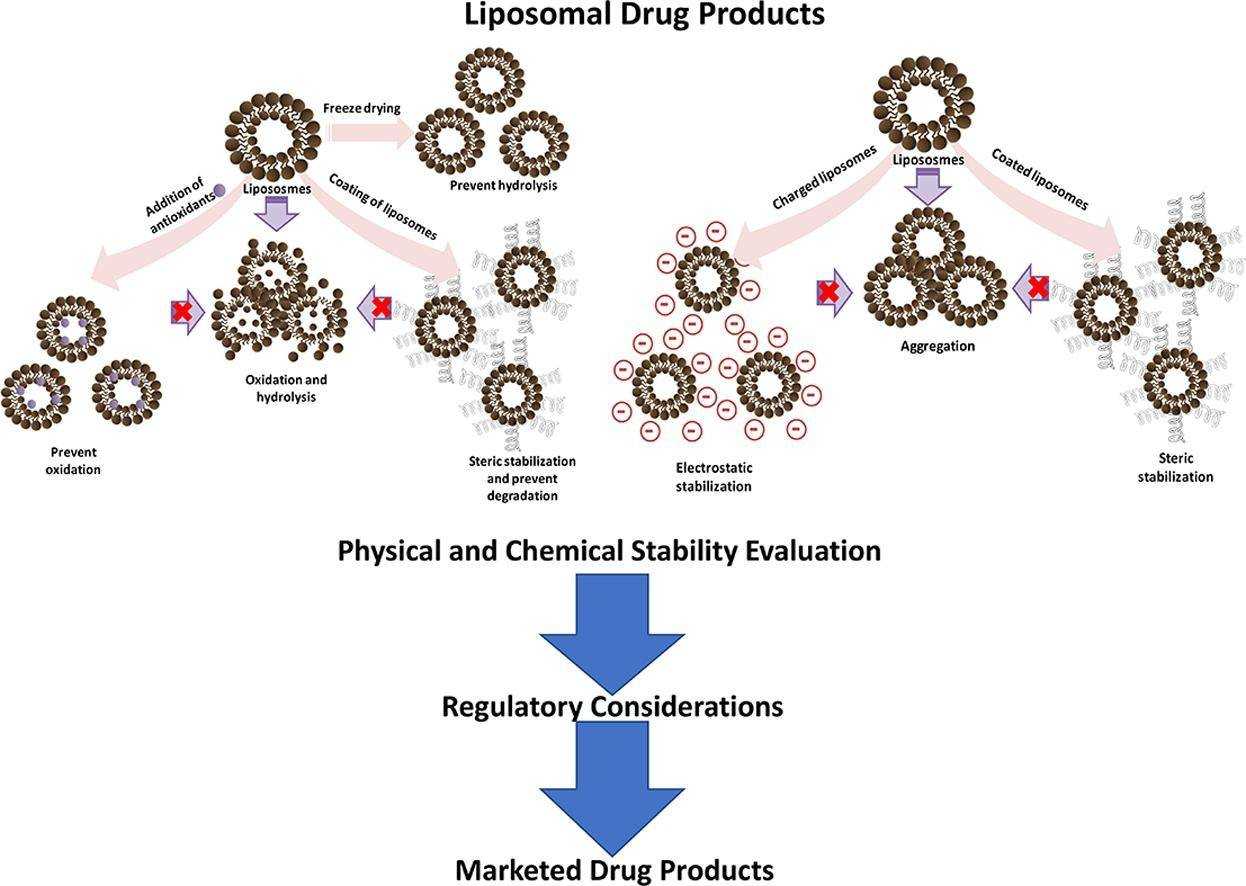 Fig.1 Stability characterization for pharmaceutical liposome product development. (Jyothi VG, et al, 2022)
Fig.1 Stability characterization for pharmaceutical liposome product development. (Jyothi VG, et al, 2022)
Why is Liposome Stability and Storage Testing Needed?
Liposomes formed by phospholipid bilayers are widely used in pharmaceutical technology as a carrier for drugs and biological molecules. However, these vesicles have physical instability. Chemical instability mainly comes from lipids because their structure has functional groups that can easily oxidize and hydrolyze. The stability of lipid nanomaterials in biological fluids is important for them to work properly. Therefore, understanding biobarriers and the interactions between lipids and biological systems is essential. Consequently, conducting stability and storage experiments on liposomes is essential.
Comprehensive Liposome Stability and Storage Solutions
Chemical stability test of liposomes
This service primarily assesses the hydrolysis reactions (including pH, temperature, and buffer concentration) and oxidation reactions (specifically phospholipid oxidation) of liposomes throughout their shelf life, as well as variations in charge.
Physical stability test of liposomes
This service primarily assesses the sedimentation reaction, alterations in particle size, and drug leakage throughout the product's shelf life.
Biological stability test of liposomes
This service primarily assesses the stability of liposomes in biological samples, including blood. The evaluation criteria encompass the in vivo release profile and associated changes.
Our Capacities for Liposome Stability and Storage Studies
| Techniques and Platforms |
Specifics |
| Liposome Factorial Experiment Platform |
- Environmental factors including light, moisture, heat, acidity, alkalinity, and oxidation.
- Identifying degradation pathways and resultant degradation products.
- Reference for accelerated testing condition selection.
|
| Liposome Accelerated Stability Testing Platform |
- Validation of analytical methodologies and degradation pathways
- Reference for expiration date determination
- Usually conducted at temperatures higher than the long-term stability study temperature and under suitable humidity conditions.
|
| Liposome Long-term Stability Testing Platform |
- Reference for determining expiration dates.
- Choosing the appropriate temperature and humidity for storage.
|
| Liposome Continuous Stability Assessment Platform |
- Physical, chemical, microbiological, and biological testing stability assessment
- Description of the container sealing system employed
- Specified storage conditions
|
Why Choose CD Formulation?
Expertise in Liposome Stability and Storage Testing
- Proficient in liposome stability and storage study.
- We are experts in liposome factorial experiment, liposome accelerated stability testing, liposome long-term stability testing, and liposome long-term stability testing.
A Powerful Stability and Storage Testing Platform
- Advanced instruments for liposome stability and storage study.
- The quality research platform is specifically designed for liposome shelf life.
A Highly Skilled Team
- We have adeptly provided invaluable support to a multitude of enterprises and academic institutions in the exploration of liposome impurities and the advancement of analytical methodologies.
- Our technical team is comprised of a diverse array of scientists who are experts in analytical chemistry and formulation development.
Published Data
Technology: microfluidic and design of experiments (DoE) methods for liposome stability studies
Journal: Drug Delivery and Translational Research
IF: 3.5
Published: 2018
Results: In this study, the author proposed a systematic approach to rapidly optimize the size of liposomes. The combination of microfluidic and design of experiments (DoE) methods provides one strategy rapid screening and optimization of various liposome formulations. Whether five representative liposome formulations based on clinically approved lipid compositions used systemically varying microfluidic flow settings, i.e., flow ratio (FRR) and total flow rate (TFR). Stability studies have shown the effects of lipid-cholesterol ratios (1:1 and 2:1 ratios) and the presence of PEG on the storage characteristics of liposomes. To validate our DoE model, we formulated liposomes containing hydrophobic dodecyl mercaptan coated gold nanoparticles. This proof conceptual step shows that the flow Settings predicted by the DoE model successfully determine the size of the resulting empty liposome (109.3±15.3 nm) or nanocomposite (111±17.3 nm).
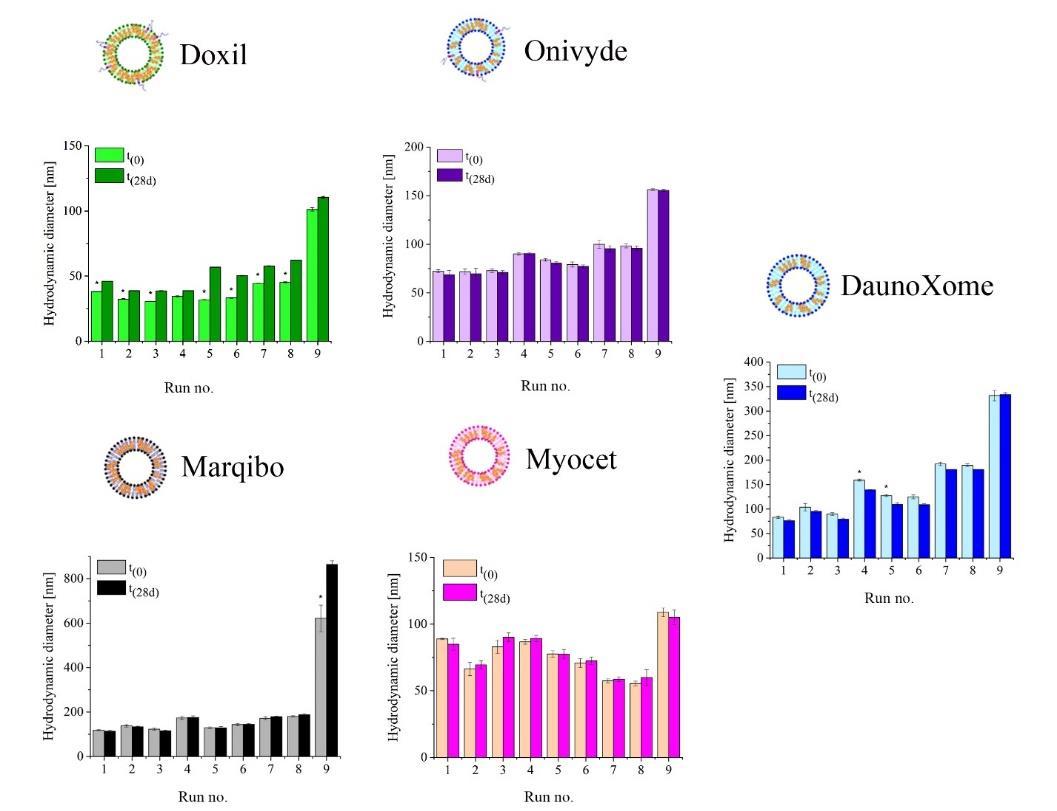 Fig.2 Storage stability of clinical liposomes formulated by microfluidics. Liposomes were stored at 4°C for 28 days. (Sedighi, Mahsa, et al. 2018)
Fig.2 Storage stability of clinical liposomes formulated by microfluidics. Liposomes were stored at 4°C for 28 days. (Sedighi, Mahsa, et al. 2018)
CD Formulation specializes in conducting comprehensive liposome stability and storage studies for pharmaceutical companies and research institutions. Our services are designed to address the unique challenges of lipid-based drug delivery systems, ensuring reliable results and actionable insights. Contact us to learn how we can support your formulation and storage research.
References
- Jyothi VG, Bulusu R, et al. Stability characterization for pharmaceutical liposome product development with focus on regulatory considerations: An update. International Journal of Pharmaceutics. 2022 Aug 25; 624: 122022.
- Sedighi, Mahsa; Sieber, Sandro; et al. Rapid optimization of liposome characteristics using a combined microfluidics and design-of-experiment approach. Drug Delivery and Translational Research. 2018. 9. 10.1007.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Stability characterization for pharmaceutical liposome product development. (Jyothi VG, et al, 2022)
Fig.1 Stability characterization for pharmaceutical liposome product development. (Jyothi VG, et al, 2022) Fig.2 Storage stability of clinical liposomes formulated by microfluidics. Liposomes were stored at 4°C for 28 days. (Sedighi, Mahsa, et al. 2018)
Fig.2 Storage stability of clinical liposomes formulated by microfluidics. Liposomes were stored at 4°C for 28 days. (Sedighi, Mahsa, et al. 2018)
