Liposome Residual Solvent Analysis
Inquiry
With the increasing complexity of lipid-based formulations, intricate active ingredient structures, and stricter safety regulations, the pharmaceutical industry faces a rising demand for thorough residual solvent analysis and method validation. CD Formulation provides specialized services to address these challenges, enabling pharmaceutical enterprises and research institutions to ensure precise and reliable analytical results to support advanced formulation development.
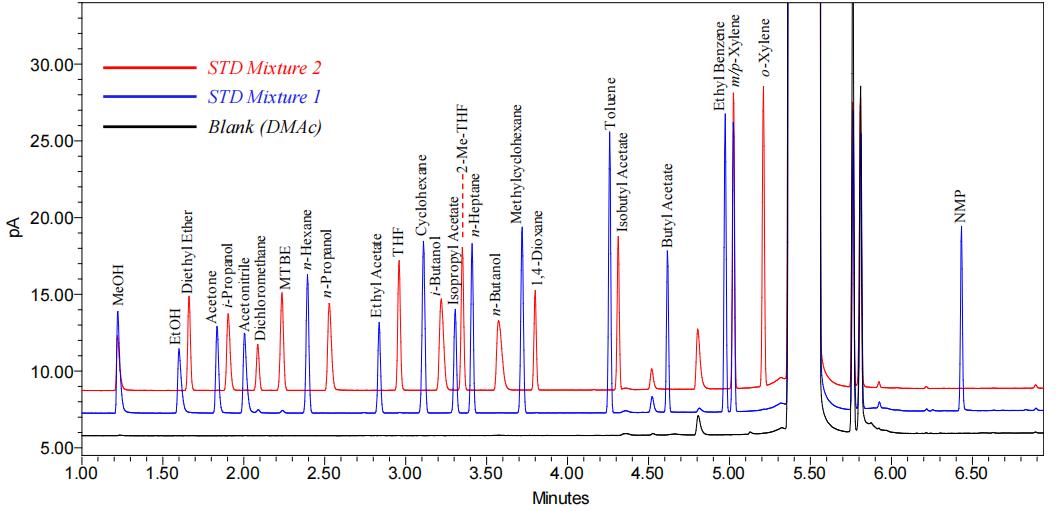 Fig.1 GC-FID profile of all solvents in mixtures 1 and 2 using DMAc as diluent. (Mann, Benjamin, et al, 2019)
Fig.1 GC-FID profile of all solvents in mixtures 1 and 2 using DMAc as diluent. (Mann, Benjamin, et al, 2019)
Why is Liposome Residual Solvent Analysis Needed?
Residual solvent analysis is a critical quality control measure. It can guarantee drugs remain free from toxic concentrations of volatile organic compounds (VOCs) throughout the manufacturing process. These VOCs may persist from previous stages or be produced during the manufacturing operations. Residual solvent detection is a critical methodology for ensuring product quality and safety. For instance, during the production of liposomes, certain organic solvents such as chloroform may be utilized. Given that drugs are either ingested or implanted into the body, testing for residual solvents is essential to confirm that their levels do not pose a risk to health. This analysis can quantify the concentration of residual solvents and assess whether they might lead to odor or taste issues, thereby safeguarding the integrity of liposomal formulations.
Comprehensive Liposome Residual Solvent Analysis Services
Selection of Analytical Methods for Residual Solvent Testing
Residual solvent determination can be performed using gas chromatography with a capillary column and headspace injection or by direct injection with a packed column. For nitrogen-containing basic compounds like N-methylpyrrolidone that are unsuitable for gas chromatography, alternative methods such as ion chromatography may be employed. Our residual solvent testing service focuses on determining the organic solvent to test, selecting the appropriate column, preparing sample and reference solutions, choosing the suitable injection method, and ensuring the detector meets sensitivity requirements.
Development and Validation of Analytical Methods for Residual Solvent Testing
At CD Formulation, we typically define the range of organic solvents to be evaluated based on the lipid formulation process, particularly for Class II or higher solvents. We also investigate the residual levels of other solvents dictated by process characteristics. Once we identify the solvents for residual analysis, we develop a feasible and robust detection method through methodological studies and perform comprehensive validation (including system suitability tests, specificity assessment, linearity evaluation, detection limit and quantitation limit determination, precision analysis, accuracy verification, and durability testing).
Our Capacities for Liposome Residual Solvent Analysis
| Techniques and Platforms |
Specifics |
| Liposome Residual Solvent Analysis Platform |
- Residual solvent analysis strategy screening
- Development of residual solvent analysis
- Transfer of methods from laboratory to laboratory and production unit
- Joint verification of personnel and instruments
|
| Instruments |
- GC-MS/MS
- GC: UV detector, fluorescence detector, etc.
- HS-GC
|
Why Choose CD Formulation?
Expertise in Liposome Residual Solvent Analysis
- Proficient in liposome residual solvent analysis methodology development.
- Support the transfer of analytical methods for residual solvent in liposome products throughout the development process, including method establishment, method validation, method transfer, and method verification.
A Powerful Analysis Platform
- Advanced instruments for liposome residual solvent.
- The quality research platform is specifically designed for liposome drugs to efficiently evaluate the residual solvent item.
A Highly Skilled Team
- We have effectively supported numerous enterprises and academic institutions in the liposome analytical method development.
- Our technical team is comprised of a diverse array of scientists who are experts in analytical chemistry and liposomes.
Published Data
Technology: headspace gas chromatography technique
Journal: Pharm Res
IF: 3.5
Published: 2020
Results: In this study, headspace gas chromatography was used to determine the residual solvent at each stage of preparation and purification. There are two solvents for the preparation of liposomes. Polymer nanoparticles prepared by nanoprecipitation and purified by ultrafiltration were studied. The effect of size exclusion chromatography and chromatography on purification was studied. Results Complete removal of residual solvent requires a process beyond conventional preparation methods. Conclusion This study can provide reference for scientists in different fields to compare their practice and simplify the transformation of nanomedicine into effective and safe drugs.
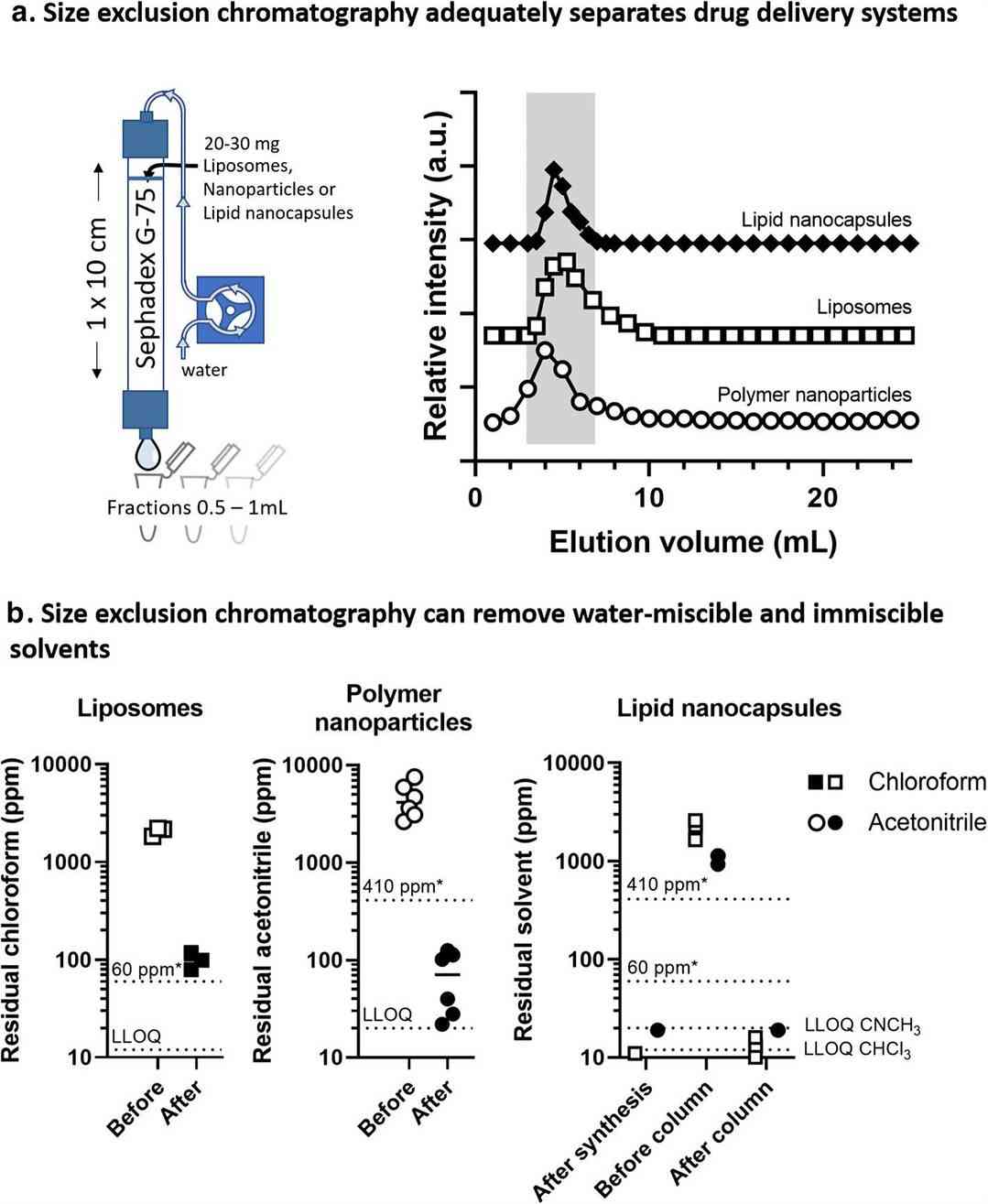 Fig.2 Size exclusion chromatography has demonstrated its efficacy as a robust technique for the elimination of residual solvents. (Dikpati, A., et al. 2020)
Fig.2 Size exclusion chromatography has demonstrated its efficacy as a robust technique for the elimination of residual solvents. (Dikpati, A., et al. 2020)
CD Formulation is dedicated to offering advanced liposome residual solvent analysis services tailored to the needs of pharmaceutical enterprises and research institutions. Our solutions help ensure the safety and efficacy of lipid-based formulations. Contact us to learn more about how we can support your analytical requirements.
References
- Mann, Benjamin & Halsey, Holst & et al. Industry-Wide Collaboration toward an Efficient and Systematic Approach to Quantitative Solvent Analysis in Drug Substances. ACS Sustainable Chemistry & Engineering. 2019. 10.1021.
- Dikpati, A., Mohammadi, F., et al. Residual Solvents in Nanomedicine and Lipid-Based Drug Delivery Systems: a Case Study to Better Understand Processes. Pharm Res; 2020; 37, 149.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 GC-FID profile of all solvents in mixtures 1 and 2 using DMAc as diluent. (Mann, Benjamin, et al, 2019)
Fig.1 GC-FID profile of all solvents in mixtures 1 and 2 using DMAc as diluent. (Mann, Benjamin, et al, 2019) Fig.2 Size exclusion chromatography has demonstrated its efficacy as a robust technique for the elimination of residual solvents. (Dikpati, A., et al. 2020)
Fig.2 Size exclusion chromatography has demonstrated its efficacy as a robust technique for the elimination of residual solvents. (Dikpati, A., et al. 2020)
