Liposome Ocular Irritation Assessment
Inquiry
Irritation refers to the reversible inflammatory response to the administration site after a non-oral drug preparation. If the administration site produces irreversible tissue damage, it is called corrosive. The stimulation test is designed to observe local reactions in the blood vessels, muscles, skin, mucous membranes, etc., following exposure of an animal to a specific substance. These reactions may include redness, swelling, edema, exudation, necrosis, or gangrene. Eye irritation tests should be considered for ophthalmic drugs and subjects that may come into contact with the eyes. Therefore, eye irritation tests play a crucial role in the quality evaluation of ophthalmic liposomes. CD Formulation provides a unique irritant evaluation platform for ocular liposomes, facilitating the development and application of ocular liposomes.
Why Conduct Liposome Ocular Irritation Assessment?
Drug ophthalmic toxicity, as a special drug toxicity, can affect the quality of life of patients and even bring serious consequences. Therefore, special attention should be paid to ocular toxicity in the process of new drug development. In vitro tests of eye injury and eye irritation are key indicators used to evaluate the safety of ophthalmic liposomes and play a key role in preclinical studies. For the research and development of ophthalmic liposomes, in the early stage of development, attention should be paid to potential eye irritation and eye injury responses based on comprehensive non-clinical safety evaluation, and optimization should be carried out from the aspects of action targets, mechanism pathways, and tissue distribution to obtain ophthalmic liposomes with minimal risk of ocular toxicity, and the benefit/risk ratio of clinical use should be evaluated through non-clinical studies. This is crucial for subsequent clinical trials and even post-market applications of the product.
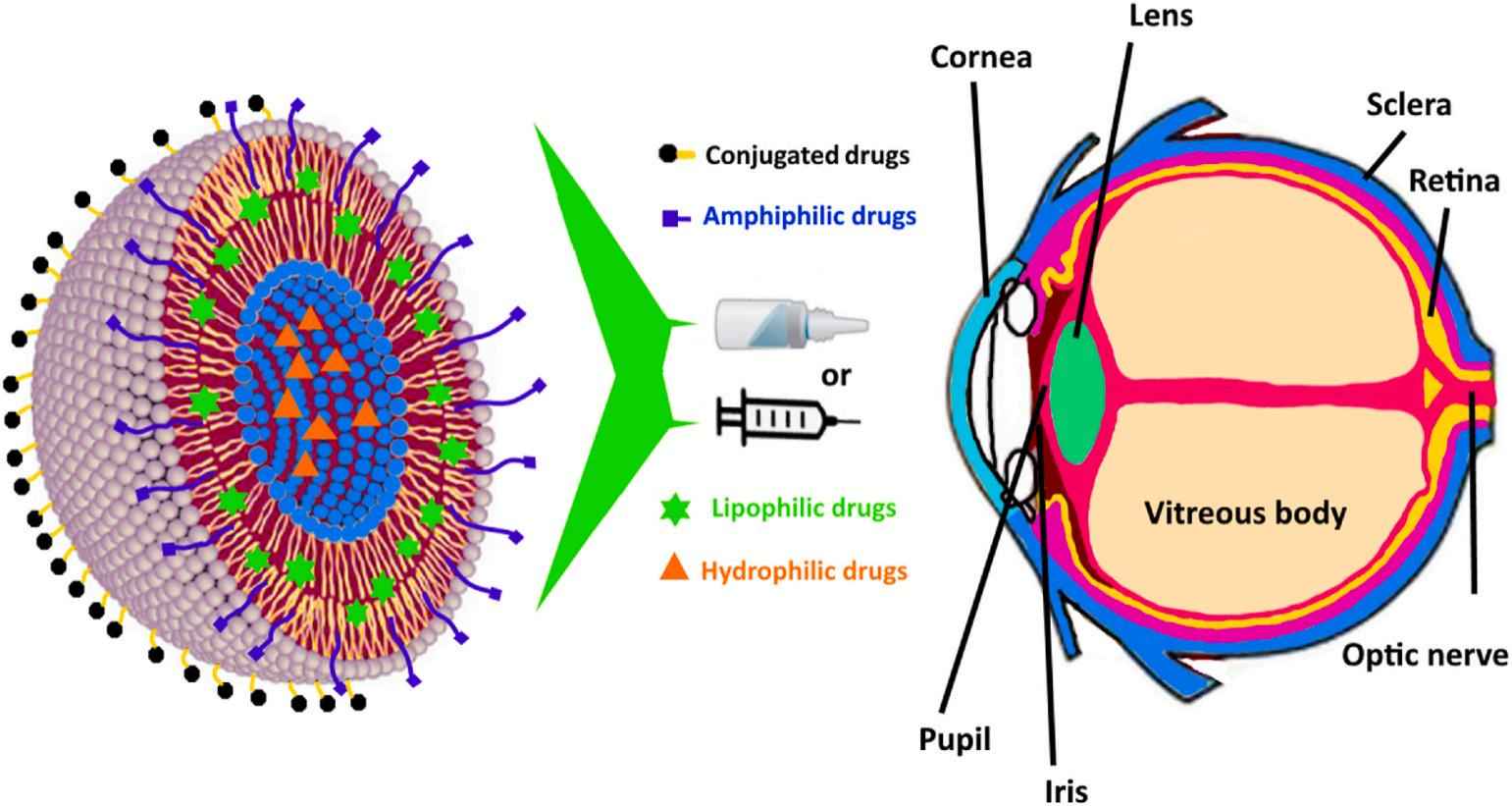 Fig.1 Illustration of ocular drug delivery after topical administration. (Nooshin Tasharrofi, et al., 2022)
Fig.1 Illustration of ocular drug delivery after topical administration. (Nooshin Tasharrofi, et al., 2022)
Our Services for Liposome Ocular Irritation Assessment
Draize Eye Irritation Test
We provide the industry-leading standard for assessing eye irritation through the in vivo Draize eye irritation test.
Alternative In Vitro Methods for Ocular Irritation Assessment
We offer the following selection options to our customers:
- 3D corneal tissue models
- Chorioallantoic membrane vascular assay (CAMVA) method
- Bovine corneal opacity and permeability assay (BCOP)
- Isolated chicken eye (ICE) test method
- Reconstructed Human keratoid Epithelial Eye Stimulation Test (RhCE EIT)
- The fluorescein leakage (FL) test method
Eye Tissue Collection and Sample Processing
Eye tissues vary in size, tissue structure, and properties, and tissue treatment methods vary. We provide various equipment such as shear homogenizers, ball mills, and multi-functional biological sample homogenizers for processing.
Ophthalmologic Examination
After the completion of the ophthalmic administration operation, in addition to collecting samples for analysis, it is often necessary to pay close attention to whether there is any abnormality in the eye after the administration of the drug, which requires detailed examination and evaluation by an experienced veterinarian with the help of ophthalmic equipment. We have established a comprehensive eye scoring standard, which can complete systematic examination of eyes.
Our Platforms for Liposome Ocular Irritation Assessment
The platforms and technologies we can offer for liposome ophthalmic stimulation evaluation services are summarized below.
| Techniques & Platforms |
Detailed Information |
| Ophthalmologic examination techniques |
- We have established a comprehensive eye scoring standard, which can complete systematic examination of eyes.
|
| Ocular tissue harvesting and sample processing platform |
- We provide different equipment such as shear homogenizers, ball mills, and multi-functional biological sample homogenizers for processing.
|
Our Key Advantages in Liposome Ocular Irritation Assessment
- Advanced. The team is equipped with a variety of sophisticated equipment and instruments for liposome ocular irritation assessment, which can provide efficient and high-quality experimental results.
- Scientific. We establish perfect scientific research methods for each project about liposome ocular irritation assessment and scientifically verify the feasibility of each method, such as parallel experiments, repeatability, etc.
- Cost-effective. We have a large number of testing instruments and analysis platforms to ensure your test results, while the project management adopts a 1-to-1 service model to ensure efficient and fast communication.
- Skilled teams. Our in-vivo and in-vitro teams are composed of multiple scientists who possess both animal and clinical experiment qualifications and are proficient in various modes of administration of ophthalmic formulations, as well as the evaluation system for ophthalmic formulations. They are capable enough to provide support for the stimulation assessment of ophthalmic liposomes.
Published Data
Technology: Topical Ophthalmic Triamcinolone Acetonide-Loaded Liposomes technique
Journal: Pharmaceutics
IF: 5.4
Published: 2021
Results: In this study, the authors developed a topical ophthalmic liposome preparation (TALF) containing triamcinolone acetonide (TA), which, upon injection into the ocular surface, facilitates the delivery of TA to the posterior segment of the eye. To evaluate the safety, tolerability, and biological activity of TALF, animal studies and Phase I clinical trials were conducted. In addition, four patients with diabetic macular edema (DME) were treated with TALF to explore the biological activity of the agent. Mild and transient adverse events, such as dry eye and burns, were mainly reported. Current data demonstrate the safety, tolerability, and biological activity of TALF. TALF appears to be topically applied for vitreous treatment and retinal diseases responsive to TA, such as DME, thereby avoiding corticosteroid IVT and its associated hazards. To quantify TALF's eye stimulation potential, the authors evaluated conjunctival, iris congestion, swelling and discharge, congestion, and corneal opacity in rabbits.
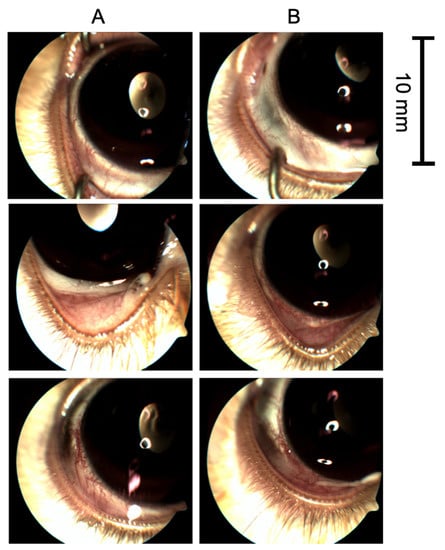 Fig.2 Representative images of study and control eyes. (Navarro-Partida J, et al., 2021)
Fig.2 Representative images of study and control eyes. (Navarro-Partida J, et al., 2021)
CD Formulation has completed the construction of an in vitro eye stimulation research platform and can provide diverse in vitro models for research. If you need any help, please do not hesitate to contact us.
References
- Nooshin Tasharrofi, Mohammad Nourozi, et al. How liposomes pave the way for ocular drug delivery after topical administration. Journal of Drug Delivery Science and Technology, 2022, 67.
- Navarro-Partida J, Altamirano-Vallejo JC, et al. Safety and Tolerability of Topical Ophthalmic Triamcinolone Acetonide-Loaded Liposomes Formulation and Evaluation of Its Biologic Activity in Patients with Diabetic Macular Edema. Pharmaceutics. 2021; 13(3):322.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 Illustration of ocular drug delivery after topical administration. (Nooshin Tasharrofi, et al., 2022)
Fig.1 Illustration of ocular drug delivery after topical administration. (Nooshin Tasharrofi, et al., 2022) Fig.2 Representative images of study and control eyes. (Navarro-Partida J, et al., 2021)
Fig.2 Representative images of study and control eyes. (Navarro-Partida J, et al., 2021)
