Liposome Ocular Drug Delivery System Development
Inquiry
Liposomes are an exceptional choice for ocular drug delivery due to their biodegradable, biocompatible nature, and ability to encapsulate a wide range of therapeutic agents. CD Formulation leverages cutting-edge technology to develop advanced liposome delivery systems for ocular drugs, supporting the R&D efforts of institutions and enterprises focused on ophthalmic treatments. Our innovative solutions are designed to enhance the precision and effectiveness of ocular drug delivery.
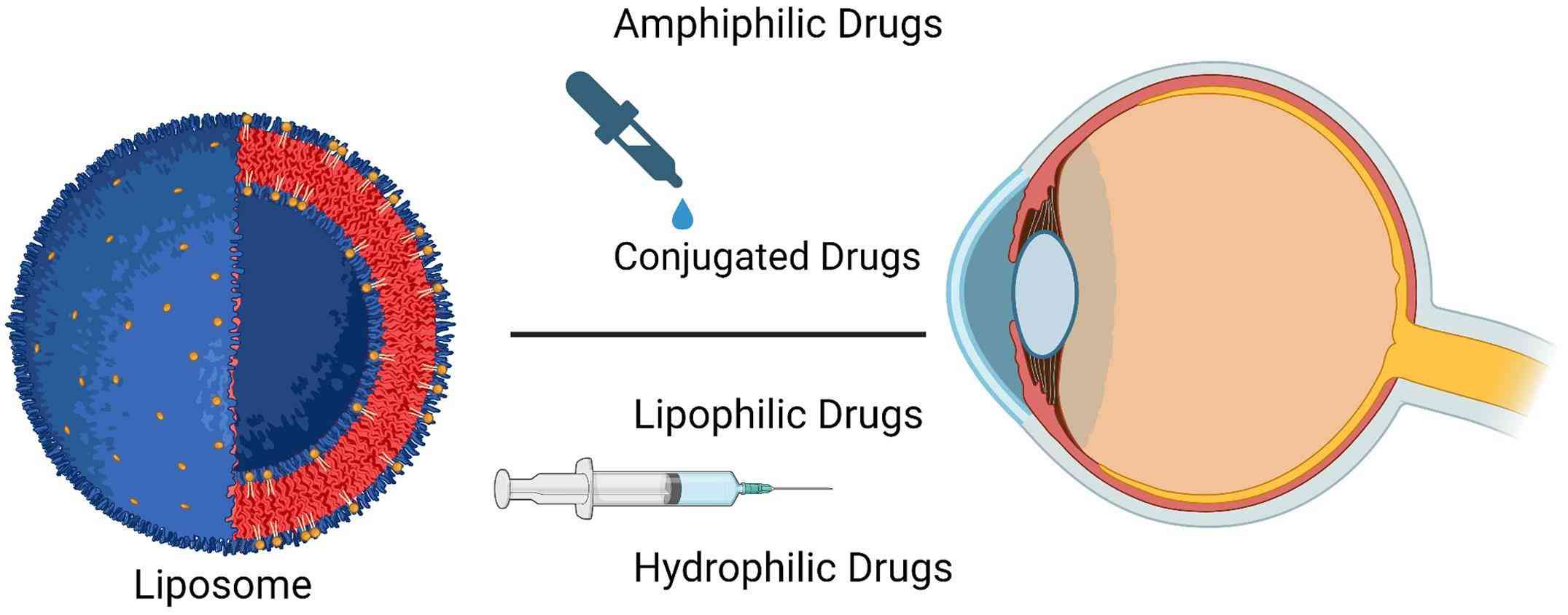 Fig.1 Liposomes delivered to the eyes. (Philip AK, 2023)
Fig.1 Liposomes delivered to the eyes. (Philip AK, 2023)
Importance of Liposome-Based Ocular Drug Delivery Systems
Eye drug delivery has many obstacles. In recent years, various innovative and reliable ophthalmic drug delivery systems have been developed. Liposomes have emerged as a prominent solution for ophthalmic drug. For example, liposomes can target the lacrimal gland via eye drops, thereby reducing collagen deposition and promoting tear secretion. These liposomes encapsulate the anti-inflammatory properties of vitamins. It creates a protective barrier that facilitates their effects while minimizing any significant impact on intraocular pressure.
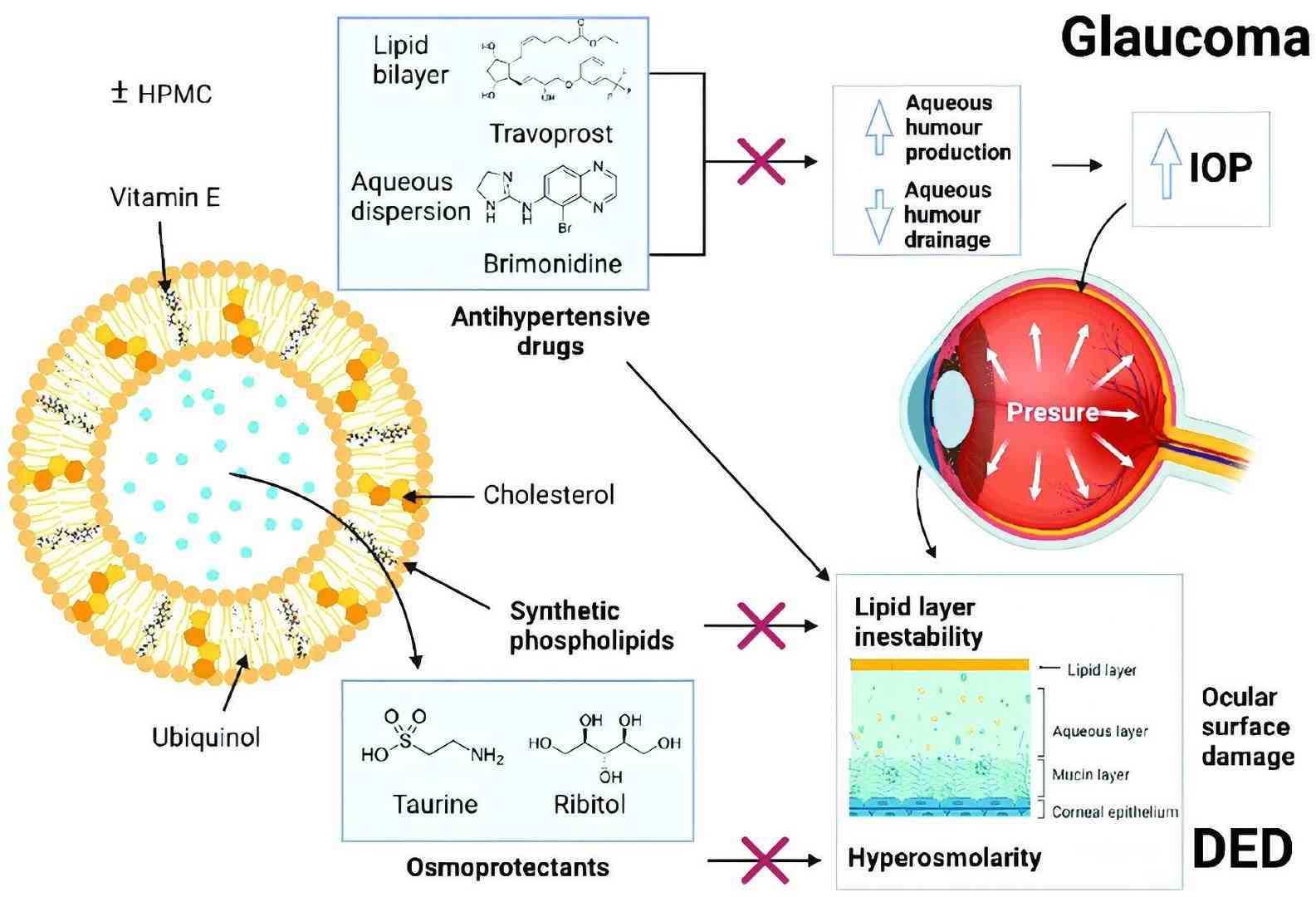 Fig.2 Liposome structure and mechanism for the treatment of glaucoma and prevention of dry eye. (Zhou, Shiqi, et al, 2023)
Fig.2 Liposome structure and mechanism for the treatment of glaucoma and prevention of dry eye. (Zhou, Shiqi, et al, 2023)
Our Liposome Ocular Drug Delivery System Development Services
Ocular Drug Delivery Route Development
By integrating liposome delivery technology, we have investigated a range of administration modalities, including local, subconjunctival, anterior chamber, vitreous cavity, retrobulbar, subtenon, posterior scleral, anterior uveal, and systemic routes.
Ocular Drug Delivery Barrier Study
The ocular barriers, which encompass the tear film, cornea, vitreous humor, blood-aqueous barrier, and blood-retina barrier, restrict the ingress of solutes and fluids into both the anterior and posterior segments of the eye. Investigating these barriers enables the development of strategies for enhancing the bioavailability of ophthalmic pharmaceuticals through liposomal formulations. Effective transgression of these barriers necessitates that the drug concentration delivered by liposomes is adequate to elicit effects while also being rapidly eliminated.
Bio-degradable and Bio-absorbable Material Study
The unique nature of ocular drug delivery makes ensuring the biodegradability and morphological suitability of the polymers in the nanocarriers critical for safe and effective drug delivery. We combine liposome technology and the development of degradable polymers to help our customers solve more degradation problems.
Characterization of Ocular Delivery Systems
Quality analysis services for ocular and periorbital local drug administration—including gels, creams, ointments, and liquid formulations such as solutions, suspensions, and emulsions—pertaining to ophthalmic drugs encompass several critical aspects: microbial contamination; visible particulates; extractables and leachables; impurities and degradation products; in vitro drug release or dissolution testing for quality assurance; design, delivery, and distribution characteristics of container sealing systems (CCS); as well as stability studies.
Our Capacities for Liposome Ocular Drug Delivery System Development
| Techniques and Platforms |
Specifics |
Liposome Drug Delivery Platform
|
- Bio-degradable and bio-absorbable material study technology
- Ocular drug delivery barrier study technology
- Capability in formulation design
- Lipid modification and formulation techniques
- Ocular drug delivery route development
|
Characterization Technology
 |
- Microbial contamination
- Visible particulates
- Extractables and leachables
- Impurities and degradation products
- In vitro drug release or dissolution testing for quality assurance
- Distribution characteristics of container sealing systems (CCS)
- Stability studies
|
Why Choose CD Formulation?
Technological Expertise in Liposome Ocular Drug Delivery System
- Our technology is suitable for different routes, including local, subconjunctival, anterior chamber, vitreous cavity, retrobulbar, subtenon, posterior scleral, anterior uveal, and systemic routes.
- Ocular drug delivery barrier study technology: tear film, cornea, vitreous humor, blood-aqueous barrier, and blood-retina barrier.
- Bio-degradable and bio-absorbable material: the polymers such as PLA, PLGA, PHA, etc.
A Powerful Evaluation Platform
- A range of sophisticated instruments for liposome characterization.
- The stability research platform supports precisely evaluating store conditions and offers verifiable data.
- The quality research platform is specifically designed for liposome ocular drugs to efficiently evaluate the characteristics of related liposomes in vivo and in vitro.
- We develop exclusive eye in vitro models to precisely assess the safety profiles of ocular drugs.
A team of Highly Skilled and Esteemed Professionals
- We have effectively supported numerous enterprises and academic institutions in the development of liposome ocular drugs.
- Our technical team is comprised of a diverse array of scientists from various disciplines, including medical science, biological engineering, materials science, and immunology.
- Our research professionals have undergone comprehensive training and certification to guarantee compliance with the highest standards of professional practice.
Published Data
Technology: F1 antigen liposome vaccine technique
Journal: Journal of Drug Delivery Science and Technology
IF: 4.5
Published: 2022
Results: In this study, the authors developed an RBM liposome eye drop formulation for the treatment of dry eye disease (DED), characterized by excellent stability, high patient compliance, and a controlled release rate. The RBM liposome eye drops were prepared utilizing remote loading technology and optimized across various formulation parameters. The optimal ratios of HSPC to cholesterol, drug-to-lipid ratio, calcium acetate solution concentration, and incubation duration were determined based on the encapsulation efficiency of the RBM liposomes. Furthermore, in vitro tolerance and therapeutic efficacy of RBM were assessed. The distribution profile of RBM within the rabbit ocular system was evaluated through in vivo studies.
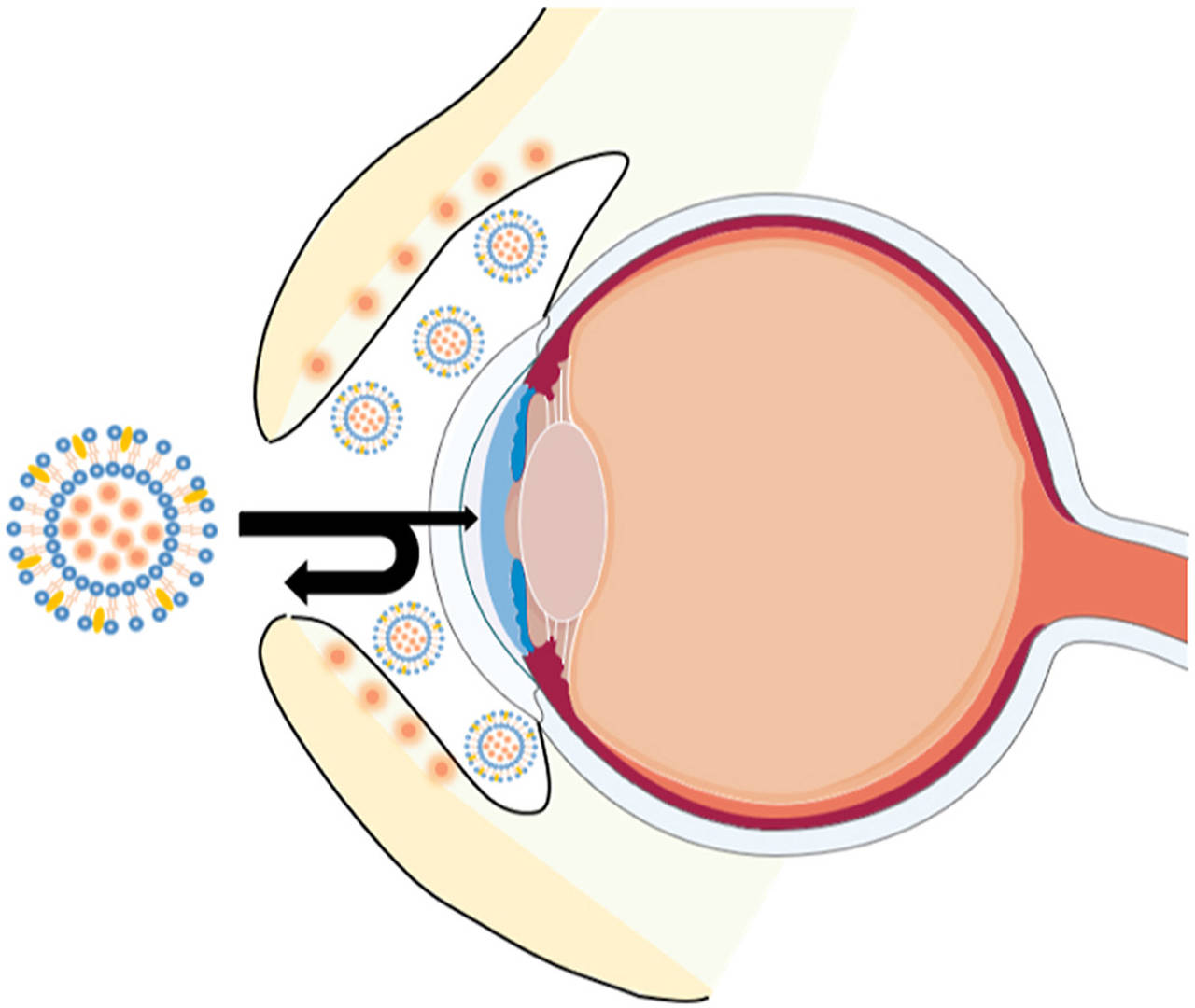 Fig.3 Illustration of rebamipide liposomes. (Qiao H, et al, 2022)
Fig.3 Illustration of rebamipide liposomes. (Qiao H, et al, 2022)
CD Formulation specializes in excellent liposome delivery technology for ocular drugs, tailored to meet the R&D needs of companies and institutions. Contact us to explore how we can contribute to your research and development projects.
References
- González-Cela-Casamayor, Miriam; Lopez-Cano, Jose; et al. Novel Osmoprotective DOPC-DMPC Liposomes Loaded with Antihypertensive Drugs as Potential Strategy for Glaucoma Treatment. Pharmaceutics. 2022. 14. 1405. 10.3390.
- Philip AK. Nanotechnology-based Therapeutic Strategies for Dry Eye Disease. J Explor Res Pharmacol. 2023; 8 (3): 253-262. doi: 10.14218.
- Qiao H, Xu Z, et al. Rebamipide liposome as an effective ocular delivery system for the management of dry eye disease. Journal of Drug Delivery Science and Technology. 2022 Sep 1; 75: 103654.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 Liposomes delivered to the eyes. (Philip AK, 2023)
Fig.1 Liposomes delivered to the eyes. (Philip AK, 2023) Fig.2 Liposome structure and mechanism for the treatment of glaucoma and prevention of dry eye. (Zhou, Shiqi, et al, 2023)
Fig.2 Liposome structure and mechanism for the treatment of glaucoma and prevention of dry eye. (Zhou, Shiqi, et al, 2023)

 Fig.3 Illustration of rebamipide liposomes. (Qiao H, et al, 2022)
Fig.3 Illustration of rebamipide liposomes. (Qiao H, et al, 2022)
