Liposome Lamellarity Analysis
Inquiry
At CD Formulation, with years of experience in liposome research, our scientists are confident that they can provide comprehensive liposome products and services through our advanced liposome platform. As a key parameter for liposomes, the degree of layering has significant and indispensable significance in the characterization process. Our layered analysis service is committed to providing high-quality services and highly competitive prices for global high-efficiency or enterprise customers.
The Importance of Liposome Lamellarity Analysis
The number of lipid bilayers in liposomes is referred to as liposome bilayers. The production methods used for liposomes can result in significant variations in their bilayer composition. Bilayers play a vital role in determining the characteristics of liposomes, such as encapsulation efficiency, regulation of drug diffusion from liposomes, control over drug release and permeability. Additionally, the fate of drugs delivered by liposomes within cells is greatly influenced by the properties of these bilayers, which can affect their absorption or processing. Therefore, accurately characterizing liposome bilayers is an essential step in preparing liposomes and a prerequisite for successful applications involving them.
What Can We Do About Liposome Lamellarity Analysis?
The layered structure plays a crucial role in defining the characteristics of liposomes; it determines the encapsulation efficiency, mediates the diffusion rate of encapsulation agents from the liposomes, controls the release of the active payload, and permeates into cells, etc. The services we are able to offer are as follows:
Analysis of liposome lamellarity assessment
This comprehensive service specialises in layered analytical testing for a wide range of liposomes to ensure the effectiveness and efficiency of your medicines.
Raw and analytical data for testing
Our data analysts and professional data processing systems will provide data processing services for you.
Our Workflow of Liposome Lamellarity Analysis
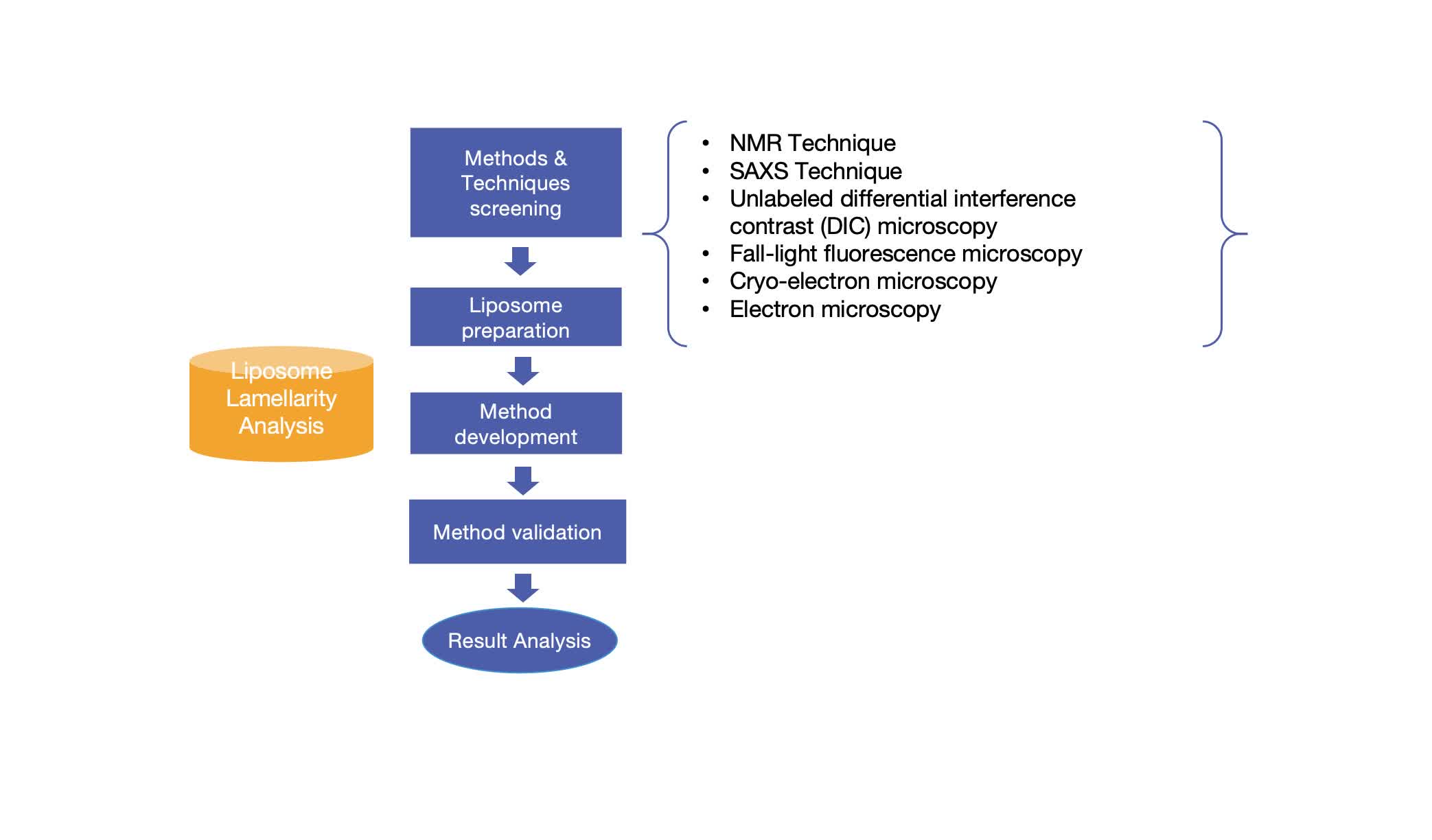 Fig.1 Workflow of liposome lamellarity analysis. (CD Formulation)
Fig.1 Workflow of liposome lamellarity analysis. (CD Formulation)
Our Technologies for Liposome Lamellarity Analysis
| Techniques & Platform |
Specifics |
| NMR Technique |
- To determine the size and lamination of liposomes.
- The signal loss caused by metal ions can be quantified after data acquisition to calculate the layeriness of the test sample.
- Due to anisotropic motion limitations, MLV produces very wide peaks on the 31 P-NMR spectrum, while SUVs tend to be less affected by this interaction.
|
| SAXS Technique |
- A widely used layering technique.
- The indirect Fourier transform provides the electron distance distribution p(r), which is calculated from the scattering intensity measured in the sample. p(r) gives the radial contrast distribution of electron density Δp (r) relative to the mean, which can be used to solve the internal structure of scattered particles.
- Accurate information about the layer of liposomes.
|
| Other Technical Platforms |
Other methods for the quantitative determination of the laminar shape of liposomes include unlabeled differential interference contrast (DIC) microscopy, fall-light fluorescence microscopy, cryo-electron microscopy, and electron microscopy. |
Our Advantages in Liposome Lamellarity Analysis
- Rapid and efficient. The entire analysis process, from the receipt of the test sample to the delivery of the analysis results, is efficiently conducted with promptness.
- Rigorous. The testing criteria of the analysis team are strictly carried out in accordance with the guidelines and are in line with science.
- Security. The confidentiality of customer projects and data protection are given priority in development.
Published Data
Technology: Dual centrifugation (DC) for the preparation of liposomes
Journal: Pharmaceutics.
IF: 5.4
Published: 2023
Results: In this study, the authors investigated the detailed structure of DC-made liposomes. For this purpose, a method for determining the ratio of the inner membrane surface to the total membrane surface (the inaccessible surface) of liposomes was developed based on time-resolved or steady-state fluorescence spectroscopy. In addition, cryo-EM was used to confirm the layered results and provide more information about the liposome morphology. A surprising result led to the possibility of producing a new type of liposome - a small multilamellar vesicle (SMV) with a low PDI, a size of about 100 nm, and almost completely filled with bilayers. The second particularly important finding was that VPG could be prepared with an open bilayer structure, which would spontaneously close when stored and additional water phase was added to form liposomes. Through this process, the drug could be effectively captured before administration. Furthermore, it was found that double centrifugation at lower lipid concentrations primarily produced monolayer vesicles.
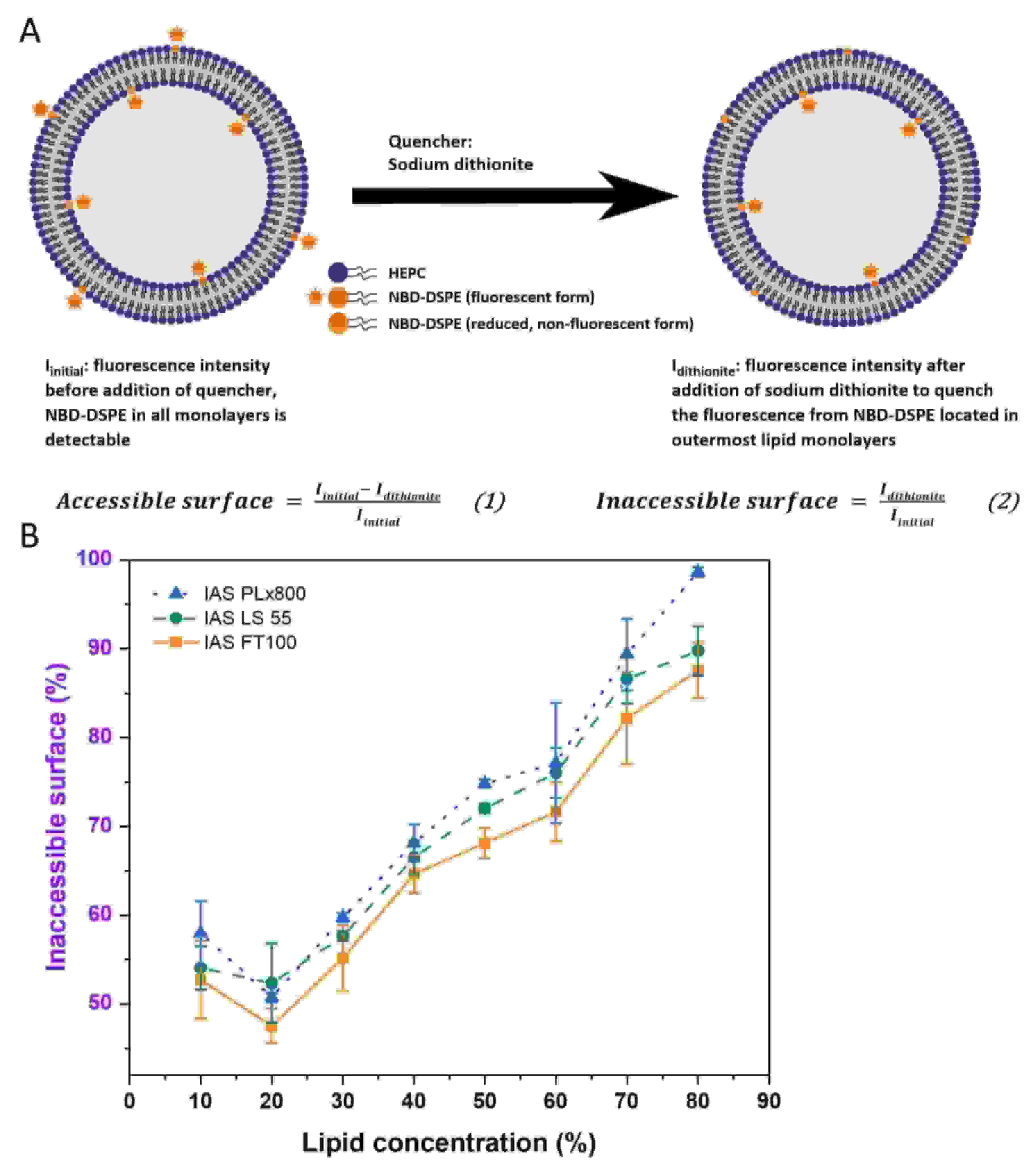 Fig.2 Schematic illustration of the inaccessible surface (IAS) assay. (Koehler, J.K., et al., 2023)
Fig.2 Schematic illustration of the inaccessible surface (IAS) assay. (Koehler, J.K., et al., 2023)
The CD Formulation team possesses extensive expertise in the evaluation of Liposome lamellarity analysis and has consistently delivered exceptional services to numerous clients, thereby earning their utmost satisfaction and trust. If you have any further inquiries, please feel free to contact us at your convenience.
References
- Koehler, J.K.; Gedda, L.; et al. Tailoring the Lamellarity of Liposomes Prepared by Dual Centrifugation. Pharmaceutics. 2023, 15, 706.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services



 Fig.1 Workflow of liposome lamellarity analysis. (CD Formulation)
Fig.1 Workflow of liposome lamellarity analysis. (CD Formulation) Fig.2 Schematic illustration of the inaccessible surface (IAS) assay. (Koehler, J.K., et al., 2023)
Fig.2 Schematic illustration of the inaccessible surface (IAS) assay. (Koehler, J.K., et al., 2023)
