Liposome In Vitro Transdermal Diffusion Study
Inquiry
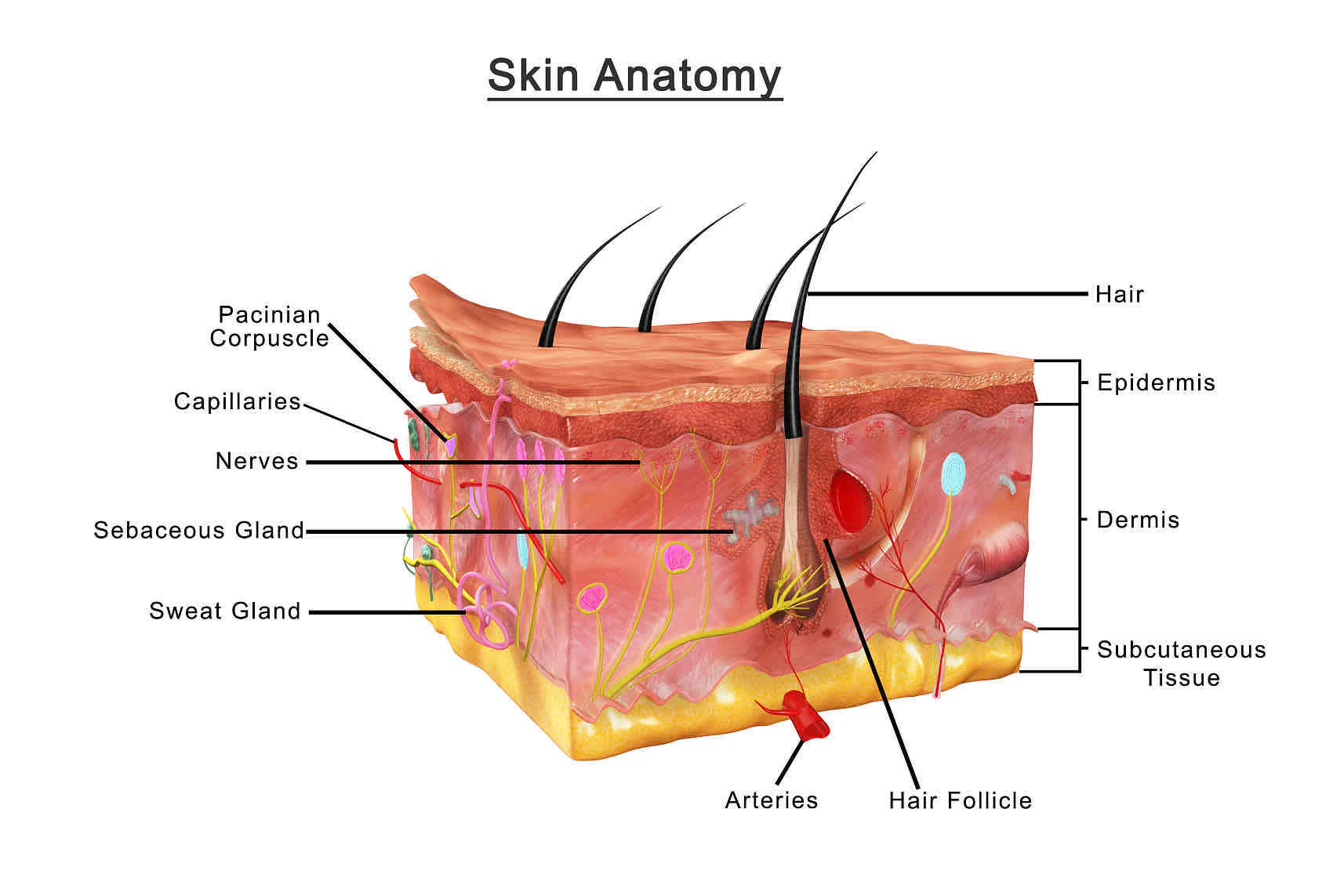
Transdermal preparation refers to the dosage form that is administered through the skin surface, so that the drug passes through the skin at a constant speed or near a constant speed, and then absorbs into the systemic circulation to play a local or systemic therapeutic effect. Since the drug does not pass through the gastrointestinal tract, the transdermal drug delivery system has the advantages of avoiding the influence of pH and transport enzymes in the digestive tract, and avoiding the first pass effect of the liver, ensuring the drug concentration and controlling the drug delivery speed, so as to maintain a constant blood drug concentration and reduce the frequency of administration, thereby improving the adaptability of patients. It has a good application prospect. The CD Formulation analysis center is capable of providing comprehensive, professional skin diffusion studies for liposomal transdermal products.
Why You Need Liposome In Vitro Transdermal Diffusion Study?
A drug in a transdermal preparation can only be effective after it enters the systemic circulation through the skin. Skin is the rate-limiting barrier for transdermal absorption of drugs, so the efficiency of the drug passing through the skin directly affects the quality and effectiveness of the preparation. Therefore, the drug diffusion efficiency is not only an important indicator of the quality control of the preparation, but also an internal guarantee of the effectiveness of the drug.
Explore Our Services for Liposome In Vitro Transdermal Diffusion Study
Screening of Transdermal Diffusion Devices
At present, there are 3 kinds of transdermal diffusion experimental devices provided by us, which are horizontal diffusion cell Valia-Chien cell, vertical Franz diffusion cell and flow cell. Franz vertical diffusion cell is the main method.
Selection of Absorbing Medium
Our absorption medium is specifically formulated to closely mimic bodily fluids, such as physiological saline or a pH 7.5 phosphate buffer solution. In the event that the drug exhibits limited solubility in these media, there is a possibility of saturation during diffusion, resulting in decelerated or halted transdermal penetration. Under such circumstances, it is recommended to substitute the absorption medium with fresh media or incorporate suitable surfactants.
Selection of Skin Types
We provide human skin, animal skin, artificial composite membrane for customers to choose.
Our Platforms for Liposome In Vitro Transdermal Diffusion Study
| Platforms |
Specifics |
| Screening of Transdermal Diffusion Platforms |
- Choriallantoic membrane vascular assay (CAMVA)
- Bovine corneal opacity and permeability assay (BCOP)
- Isolated chicken eye (ICE) test method
- Reconstructed Human keratoid Epithelial Eye Stimulation Test (RhCE EIT)
- fluorescein leakage (FL) test method.
|
| Analysis Platform |
- Fourier TransformInfrared Spectroscopy
- CoherentAnti-Stokes Raman Scattering
- Surface-enhanced Raman Scattering
- Stimulated Raman Scattering
|
Highlights for Liposome In Vitro Transdermal Diffusion Study
- Advanced techniques. We have our screening of transdermal diffusion platforms and we combine multiple technologies such as: CAMVA, ICE test, RhCE EIT, FL test method, etc.
- Unique. Independent project management system for liposome in vitro transdermal diffusion study, each project has its own folder.
- Efficiency. The study reports and results are delivered to customers quickly and efficiently throughout the process.
- Top equipment. Our lab is equipped with fourier transformInfrared spectroscopy, coherent Anti-Stokes raman scattering, surface-enhanced raman scattering, stimulated raman scattering, and so on.
Published Data
Technology: transdermal delivery of nonsteroidal anti-inflammatory drugs technique
Journal: ACS Omega
IF: 10.5
Published: 2022
Results: In this study, a novel mixed liposomes (CTAB, IA-16(OH), IAC-16(Bu), and PR-16) with different cationic head group structures were developed for transdermal delivery of nonsteroidal anti-inflammatory drugs. Different combinations of surfactants/lipids were studied to obtain highly functional stable liposomes. The hydrodynamic diameter of the cationic liposomes was ~110 nm. The combination of cationic surfactants and PC lipids improved the physical and chemical properties of the vesicles and enhanced transdermal diffusion rate and prolonged the release of the drug. The transdermal permeation of liposome-encapsulated diclofenac sodium (DS) and ketoprofen (KP) was tested using Franz cells. The 48-hour drug diffusion monitoring showed that the maximum DS and KP permeability through the synthetic membrane (Strat-M) were 255±2 and 186 ± 3 μg/cm2, respectively. This study demonstrates for the first time the influence of the head group of the surfactant on the properties (stability, release profile, permeability) of cationic liposomes. Although the drug-specific release rate was clearly evident, the permeability increased as follows: conventional liposomes < CTAB/PC < PR-16/PC < IAC-16(Bu)/PC < IA-16(OH)/PC. The in vivo anti-inflammatory effect of the IA-16(OH)/PC lead formulation was evaluated using a rat paw edema model. It was found that liposomes DS and KP could effectively alleviate edema of the rat paw. It is worth noting that compared with traditional vesicles, the mixed liposomes loaded with DS showed the highest therapeutic efficacy.
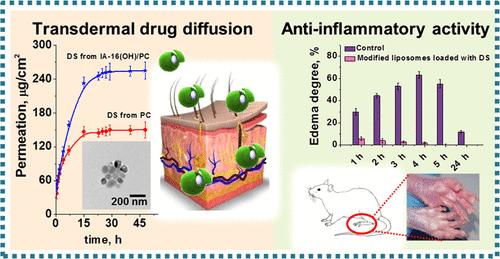 Fig.1 Evaluation of nonsteroidal anti-inflammatory liposomes. (Darya A. Kuznetsova, et al., 2022)
Fig.1 Evaluation of nonsteroidal anti-inflammatory liposomes. (Darya A. Kuznetsova, et al., 2022)
CD Formulation's liposome in vitro transdermal diffusion study service is designed to provide comprehensive analytical services to our clients. If you need any kind of assistance, please contact us immediately.
References
- Darya A. Kuznetsova, Elmira A. Vasilieva, et al. Enhancement of the Transdermal Delivery of Nonsteroidal Anti-inflammatory Drugs Using Liposomes Containing Cationic Surfactants. Sinyashin. ACS Omega. 2022. 7 (29), 25741-25750
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services



 Fig.1 Evaluation of nonsteroidal anti-inflammatory liposomes. (Darya A. Kuznetsova, et al., 2022)
Fig.1 Evaluation of nonsteroidal anti-inflammatory liposomes. (Darya A. Kuznetsova, et al., 2022)
