Liposome Freeze-thaw Testing
Inquiry
Liposomes often require cold chain transportation at 2–8°C. However, during winter transport, ambient temperatures may drop below freezing, triggering the "freeze-thaw cycle." This phenomenon can lead to two major risks: physical damage, such as shattering of glass vials, which causes contamination, and functional issues, including diminished efficacy or toxic mutations of the liposome formulation. CD Formulation leverages cutting-edge testing methodologies to assess and mitigate these risks, ensuring the reliability of liposomal drug delivery systems.
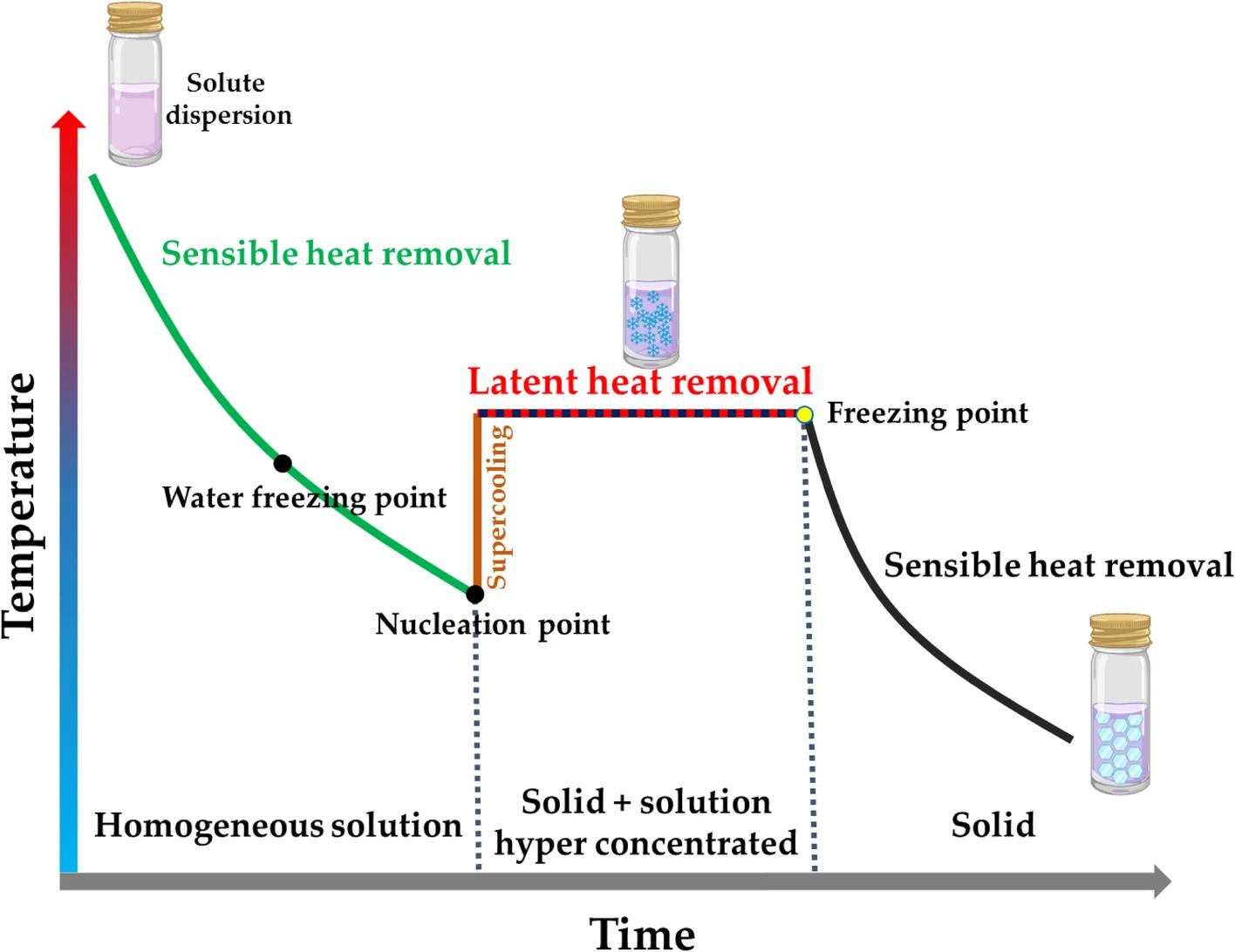 Fig.1 Typical freeze-thaw time-temperature curve. (Bernal-Chávez, S.A, et al, 2023)
Fig.1 Typical freeze-thaw time-temperature curve. (Bernal-Chávez, S.A, et al, 2023)
Importance of Freeze-Thaw Testing for Liposome Stability
The freeze-thaw cycle of pharmaceuticals pertains to a physicochemical phenomenon and condition induced by the processes of freezing and thawing, which occur when temperatures fall below or rise above zero degrees Celsius. This cyclical process can lead to alterations in the properties of pharmaceutical compounds, potentially compromising their activity or efficacy. The stability assessment of liposomal formulations primarily encompasses the evaluation of influencing factors, accelerated testing, and long-term stability studies. Given that liposomal drugs have specific temperature requirements, it is essential to conduct freeze-thaw cycle tests. The low-temperature and freeze-thaw assessments should consist of three cycles: a 2-day exposure at low temperatures or during freeze-thaw conditions, followed by 2 days at an appropriate ambient temperature.
Comprehensive Liposome Freeze-Thaw Testing Solutions
Freezing Point Study of Liposome Drugs
The freezing points of various pharmaceuticals differ, which influences the temperature and duration of the freeze-thaw experiment. We provide specialized freezing point research services for liposomal drugs.
Freeze-thaw Testing Condition Screening
In general, for pharmaceuticals with a temperature range above the freezing point, the thermal cycling test we offer encompasses three cycles, each lasting between 2 to 8 days at temperatures of 2 to 8°C, followed by a two-day evaluation period at 40°C under accelerated conditions. For pharmaceuticals that may be subjected to temperatures below the freezing point, the thermal cycling test we offer also consist of three cycles, with each cycle maintained for 2 days at -10 to -20°C, succeeded by a two-day assessment at 40°C under accelerated conditions. In the case of inhalation aerosols, our thermal cycling test includes three to four six-hour cycles within a single day, with temperature fluctuations ranging from below freezing up to 40°C (75-85% relative humidity). For drugs stored at low temperatures, their stability must be evaluated when they are rapidly thawed using either microwave or hot bath methods unless explicitly stated otherwise in the product instructions.
Multiple Verification and Data Analysis
We provide professional and systematic data support for liposome freeze-thaw cycle research experiments and provide verifiable data support for customers through repeated experiments.
Our Capacities for Liposome Freeze-thaw Testing
| Techniques and Platforms |
Specifics |
| Freeze-thaw Techniques |
- Temperature, humidity, time, frequency of screening
- Curve study
- Freezing point study
- Effects of freeze-thaw on physicochemical properties of liposome samples
|
| Analysis Platform |
- Size
- Potential
- Condensation phenomenon
- The rupture of liposomes and the changes in the encapsulation rate
|
Why Choose CD Formulation?
Liposome Freeze-thaw Testing Techniques
- Proficient in liposome freeze-thaw testing
- Free-thaw curve study
Powerful Freeze-thaw Testing Platforms
- Advanced freeze-thaw equipment and thermostat
- Advanced analytical facilities ensure the study of physicochemical properties of liposomes.
A Great and experienced Team
- Successfully provided vital support to numerous corporations and academic institutions, enabling them to delve deeply into liposome stability after freeze-thaw cycle.
- We have a great team for liposome stability and physical and chemical properties.
Published Data
Technology: camera-assisted methodology and optimized methodology for liquid sampling after thawing technique
Journal: Methods and Protocols
IF: 2.3
Published: 2024
Results: In this study, the authors report the optimization of two main methods for studying process parameters that are independent of the product at manufacturing scale, namely recording temperature curves and sampling liquid after thawing to quantify the concentration gradient in the solution. They report the experimentally proven measurement positions for temperature recording, particularly the selection of the final freezing point position, and emphasize the implementation of camera-assisted inspection to determine the actual thawing point and actual thawing time. They provide a detailed description of the technical implementation of these measurement devices for the first time. Based on the case study reported, we suggest selecting relevant measurement positions based on the initial equipment characteristics to form a resource-intensive research setup.
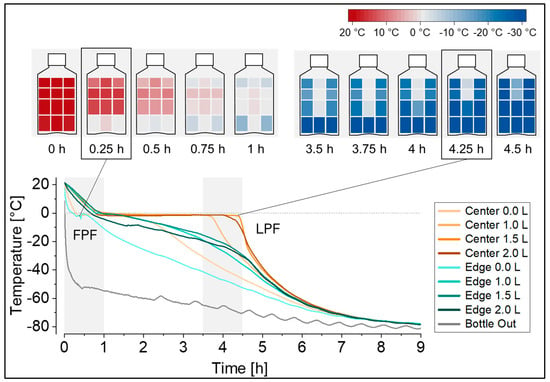 Fig.2 Temperature profiles during freezing at −80°C recorded at eight temperature probe positions in the 2 L DS bottle filled with 2 L of surrogate solution. (Peláez SS, et al. 2024)
Fig.2 Temperature profiles during freezing at −80°C recorded at eight temperature probe positions in the 2 L DS bottle filled with 2 L of surrogate solution. (Peláez SS, et al. 2024)
CD Formulation specializes in studying the impact of freeze-thaw cycles on liposome stability. Our tailored services help pharmaceutical companies and research institutions maintain the quality and safety of lipid-based formulations under variable temperature conditions. Contact us to learn how we can support your research and development needs.
References
- Bernal-Chávez, S.A., Romero-Montero, A., et al. Enhancing chemical and physical stability of pharmaceuticals using freeze-thaw method: challenges and opportunities for process optimization through quality by design approach. J Biol Eng 17, 2023, 35.
- Peláez SS, Mahler H-C, et al. Optimization of Methodologies to Study Freeze/Thaw Processes in Drug Substance Bottles. Methods and Protocols. 2024; 7(5): 68.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Typical freeze-thaw time-temperature curve. (Bernal-Chávez, S.A, et al, 2023)
Fig.1 Typical freeze-thaw time-temperature curve. (Bernal-Chávez, S.A, et al, 2023) Fig.2 Temperature profiles during freezing at −80°C recorded at eight temperature probe positions in the 2 L DS bottle filled with 2 L of surrogate solution. (Peláez SS, et al. 2024)
Fig.2 Temperature profiles during freezing at −80°C recorded at eight temperature probe positions in the 2 L DS bottle filled with 2 L of surrogate solution. (Peláez SS, et al. 2024)
