
CD Formulation has extensive experience in liposome formulation screening and can provide screening services for various systems to develop liposome formulations across multiple industries. Our professional team tailors liposome formulation screening solutions according to your needs, helping you quickly find the most suitable formulation for your project. We offer a wide range of services in drug delivery, biomedical applications, cosmetics, and food industries. Equipped with advanced equipment and skilled professionals, our technical center ensures high-quality results during the efficient process of liposome screening and characterization. Whether it's liposome preparation, drug delivery, biomedical applications, or cosmetics and food industry development, we provide expert advice and support for the success of your project.
Why Do You Need Liposome Formula Screening?
Have you ever considered the intricate process involved in developing an optimal liposome formulation? The reality is that it entails more than mere blending of various ingredients together. To ensure the efficacy and safety of your formula, it must undergo a series of rigorous assessments and evaluations. This is where the utility of liposome formulation screening services becomes apparent. These services encompass a comprehensive evaluation procedure aimed at assessing the safety and effectiveness of your liposome formulation. This process encompasses diverse screening procedures, including drug-to-lipid ratio, preparation method, loading technique, buffer solution type and proportion, API dosage, water phase to oil phase ratio, etc. Such screenings are indispensable for identifying any potential issues with the formulation such as instability, reactivity or toxicity before they manifest problems in practical applications.
Our Liposome Formula Screening Services
Our state-of-the-art facility is a cutting-edge laboratory equipped with the latest technology and advanced instrumentation, providing a comprehensive suite of services tailored to screen liposome formulations. The screening process at our facility is designed to encompass a diverse range of procedures, ensuring the highest level of accuracy and efficiency. Moreover, we also take into account factors such as the API dosage, water phase to oil phase ratio, and other critical parameters that impact the quality and stability of liposome formulations.
Our capabilities are as follows, but are not limited to:
- Drug-to-lipid ratio screening. The drug-lipid ratio, abbreviated as DLR, is evaluated using encapsulation rate and particle size as evaluation indicators to assist customers in selecting an initial suitable formula.
- Buffer solution type and proportion screening. Using leakage rate or transmission electron microscopy images as indicators, the type of buffer solution can be evaluated to help control the internal aqueous phase and outer aqueous phase buffering capacity.
- Type and ratio screening of excipients. In this service, we help clients to compare the type and proportion of excipients are compared such as phospholipids, cholesterol, amino acids, and so on.
- Oil phase ratio screening. The service assists customers in determining the oil phase ratio and type in the formulation by conducting a comparative analysis of liposome stability and particle size.
Our Workflow of Liposome Formula Screening
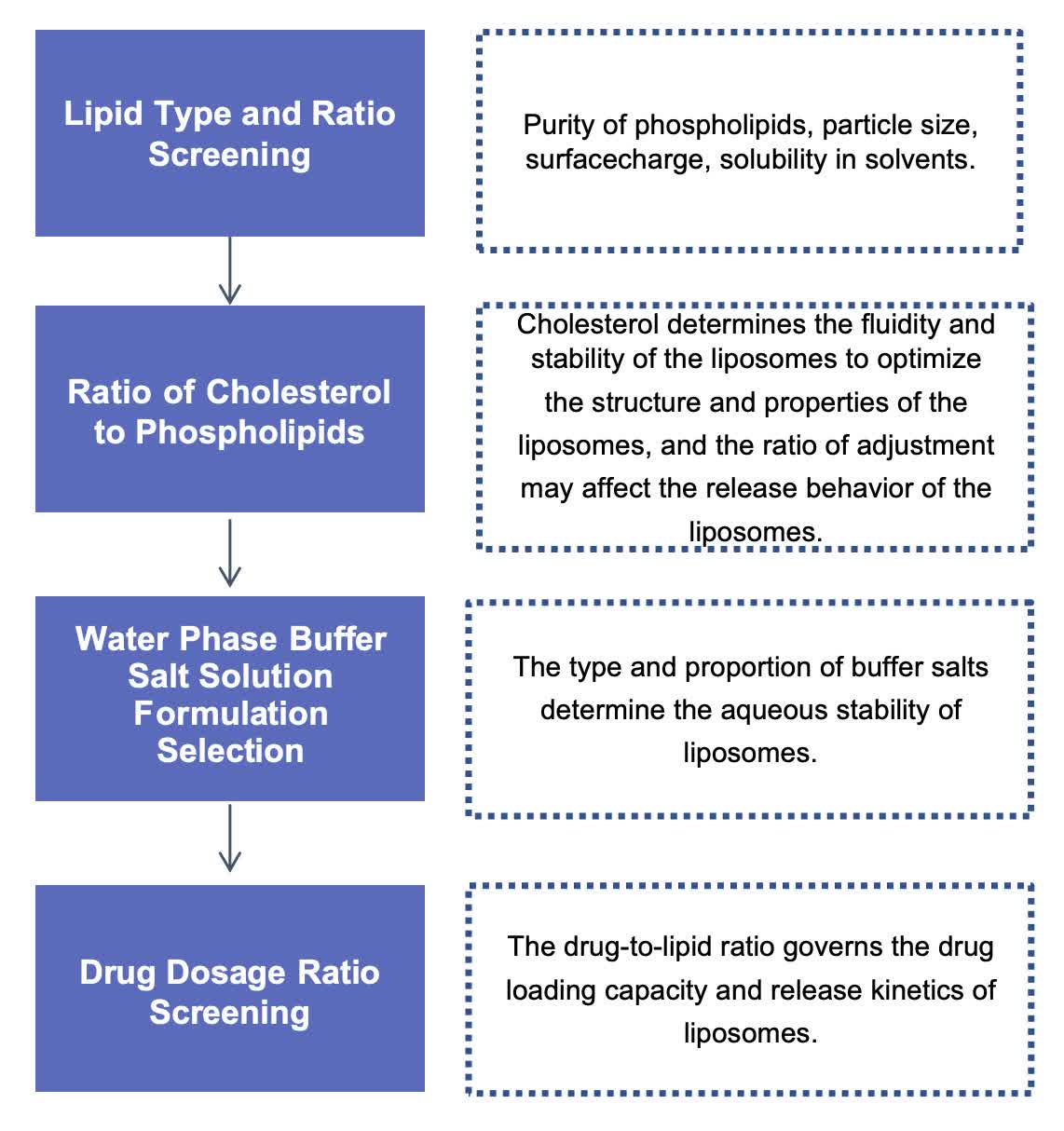 Fig.1 The workflow of our liposome formula screening services. (CD Formulation)
Fig.1 The workflow of our liposome formula screening services. (CD Formulation)
Our Platform for Liposome Formula Screening
| Platform Equipment |
Specifics |
Liposome Homogenizer
 |
- Simple manual or automatic control interface.
- Integrated variable-speed drive for smooth and flexible operation over a range of flow rates.
- Triple-plunger design enhances flow rate stability.
- Modular design for use in laboratories to continuous production.
- Direct-drive variable-speed gearbox reduces maintenance.
- Integrated controls simplify installation and reduce costs.
|
Transmission Electron Microscopy (TEM)
 |
- Magnify specimens to a greater extent than with light microscopy.
- Magnification of 10,000 times or more is possible, which allows scientists to see very small structures.
- For biologists, the inner workings of cells, such as mitochondria and organelles, are clearly visible.
- The crystal structure of the TEM specimen provides excellent resolution and can even show the arrangement of atoms within the sample.
|
Laser scanning Confocal Microscopy
 |
- Precision 3D measurement.
- Measurement results can be traced.
- Non-contact measurement.
- Accurate positioning measurement.
- High-definition color observation
|
High-performance Liquid Chromatography (HPLC)
 |
- Highly efficient and rapid.
- Good accuracy.
- Versatile and extremely accurate in identifying and quantifying chemical compositions.
- Determination such as drug loading, encapsulation rate, leakage rate, lipid content, etc.
|
Why Choose Us to Screen Liposome Formula?
- Enhanced expertise. Our team of professionals possesses extensive knowledge and a wealth of experience, enabling us to conduct comprehensive evaluations across various domains.
- Advanced technology utilization. By harnessing the power of cutting-edge analysis and evaluation platforms, we effectively cater to the diverse analytical needs of our esteemed clientele.
- Tailored solutions. Our services are highly adaptable and can be personalized to meet your specific requirements.
- Stringent quality control measures. We enforce rigorous quality control protocols throughout every stage of development and manufacturing processes.
- Optimal efficiency. Through efficient project management and streamlined procedures, we ensure maximum productivity levels are achieved.
Published Data
Technology: Liposomes development process with Ishikawa diagram and failure mode effects analysis
Journal: Pharmaceutics
IF: 5.0
Published: 2021
Results: In this study, Isikawa diagram was used as a risk assessment tool to outline material properties and process parameters that could have a key impact on SIM-DOX-LCL CQAs. In view of the increase in the number of factors identified, another risk analysis method, FMEA, was also employed. Using FMEA, each CQA (i.e., drug encapsulation concentration, EE%, liposome size, PdI, and ζ potential) was evaluated in terms of failure effects, potential causes, and control methods.
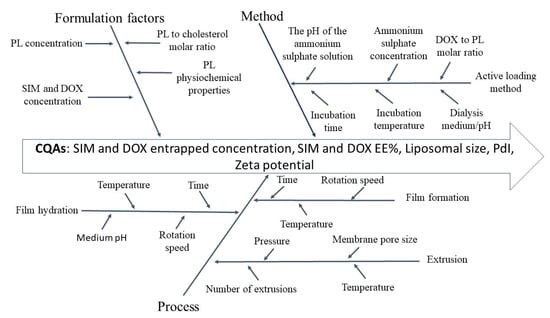 Fig.2 Liposomes process evaluation with Ishikawa diagram. (Barbălată, C.I., et al., 2020)
Fig.2 Liposomes process evaluation with Ishikawa diagram. (Barbălată, C.I., et al., 2020)
The comprehensive and professional formulation screening services provided by CD Formulation are backed by advanced technology and equipment. If you have any specific requirements, feel free to contact us, and our team is readily available for consultation.
References
- Barbălată, C.I.; Porfire, A.S.; et al. A Screening Study for the Development of Simvastatin-Doxorubicin Liposomes, a Co-Formulation with Future Perspectives in Colon Cancer Therapy. Pharmaceutics, 2021, 13, 1526.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services









