Liposome Encapsulation Service for siRNAs
Inquiry
siRNA has emerged as a powerful tool in therapeutic research, effectively targeting gene overexpression and mutations. Its applications span various fields, including studies on viral infections, cancer, genetic disorders, and autoimmune diseases. CD Formulation specializes in providing advanced liposome encapsulation technology for siRNA, specifically designed to meet the complex R&D requirements of pharmaceutical companies and research institutions. Our solutions empower groundbreaking advancements in gene-based research and therapeutic development.
What is siRNA?
Small interfering RNA (siRNA) is a type of double-stranded RNA. It is also known as short interfering RNA or silencing RNA. It helps in the RNA interference process and works similarly to miRNA. It interferes with the expression of specific genes with complementary nucleotide sequences by degrading mRNA post-transcriptionally, thus preventing translation.
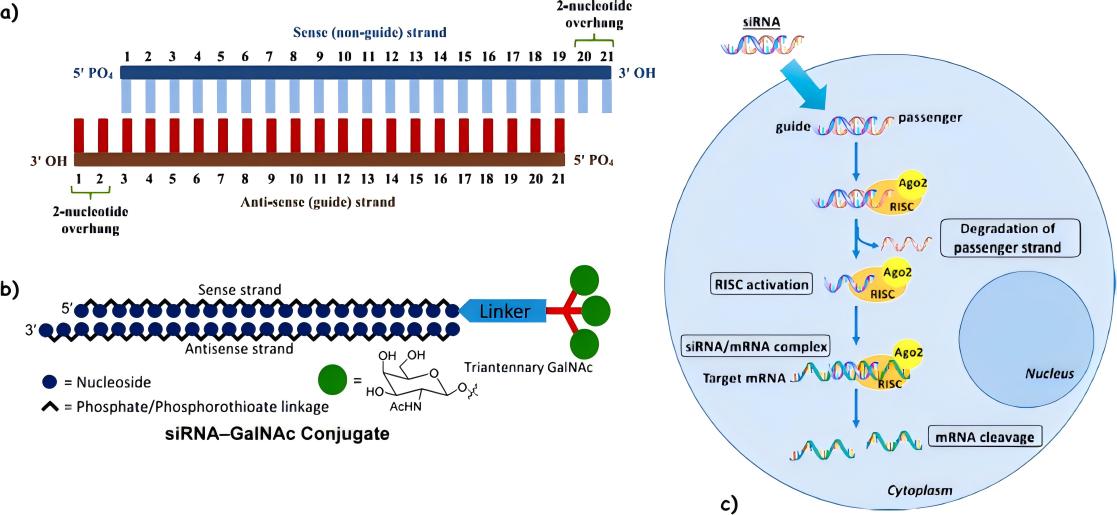 Fig.1 Schematic illustration of siRNA. (Carugo, Stefano., et al, 2023)
Fig.1 Schematic illustration of siRNA. (Carugo, Stefano., et al, 2023)
Advantages of siRNA Liposome Encapsulation
The advantages of liposome encapsulation for siRNA are mainly as follows.
- RNase and Protease Protection. Liposomes safeguard siRNA from degradation by serum RNases and proteases as it approaches its target.
- Facilitating Cellular Penetration. Liposomes enhance the ability of siRNA to penetrate cellular membranes and access the cytoplasm.
- Minimal Cytotoxicity. Liposomes exhibit relatively low toxicity to cells.
- Simplified Preparation Process. The preparation of liposomes is comparatively straightforward.
- Cost-Effectiveness. Liposomes are generally cost-effective solutions.
- Intracellular Delivery Mechanism. Liposomes facilitate the transport of target substances into cells effectively.
- Targeted Delivery System. Liposomes can be employed for the targeted delivery of siRNA to specific cell types or tissues.
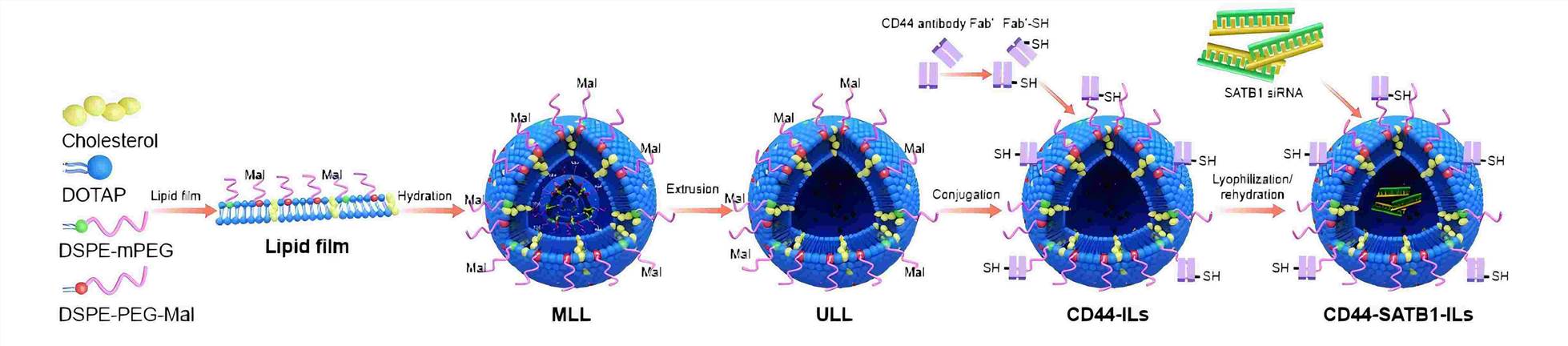 Fig.2 Liposome encapsulation for siRNA. (Yang F, et al., 2018)
Fig.2 Liposome encapsulation for siRNA. (Yang F, et al., 2018)
Our Liposome-Based siRNA Encapsulation Service
Formulation of siRNA-encapsulated liposomes
We aim to offer professional formulation services for siRNA-encapsulated liposomes. From a professional standpoint, cationic liposomes or lipids combined with anionic siRNA form self-assembled nanoparticles, primarily driven by multiple electrostatic interactions. For instance, by increasing the cationic concentration, we achieve complete complexation of the siRNA. In addition, we obtain the stable self-assembled nanoparticles by combing pre-prepared PEG-modified cationic liposomes with siRNA.
Purification Services
At CD Formulation, we help clients develop the purification method and employ a range of purification techniques to effectively eliminate unencapsulated siRNA, including the utilization of cellulose ester membrane dialysis tubes for the purification of liposomes.
Characterization service
To effectively deliver siRNA/lipid complexes to target cells, precise modulation of their size is essential to conform to the structural constraints of the target tissue. Following systemic administration, red blood cells, and endothelial cells readily interact with the siRNA/lipid complexes. We assist our clients in optimizing liposomal targeting towards specific cell types by regulating particle size.
Our Capabilities for Liposome siRNA Encapsulation
| Techniques and Platforms |
Specifics |
| siRNA Encapsulation Technology |
- Lipid formulation development.
- Lipid synthesis and modification.
- Liposome types and methods screening.
- Purification techniques.
- Size control techniques.
|
| Characterization Platform |
- In vitro evaluation techniques (such as cell uptake rate test, cytotoxicity test, release rate, and stability).
- In vivo evaluation techniques (including cell targeting test, in vivo stability, and distribution).
- Physical and chemistry evaluation (size distribution, potential analysis, and encapsulation efficiency).
|
Why Choose CD Formulation?
- Superior liposome-mediated siRNA encapsulation technology. Our siRNA liposome encapsulation technology markedly improves the bioavailability and targeted delivery of diverse siRNAs. Depending on the anticipated delivery target and site, we adeptly modify the composition and size of the liposomes to fully leverage the distinct properties of each siRNA. Furthermore, by employing advanced cationic liposome technology alongside N-acetylgalactosamine (GalNAc)-siRNA conjugation techniques, we enhance the affinity of these targeted liposomes for specific cell types or tissues, thereby facilitating advancements in precision medicine.
- Exclusive Analytical Platform. We have developed a comprehensive analysis system specifically for siRNA liposomes. This platform is equipped with a range of advanced instruments and equipment specifically designed to analyze the characteristics of liposomes carrying siRNA, providing researchers with comprehensive data support. It helps customers better understand the structural features, physical and chemical properties, and potential applications of liposomes in siRNA delivery.
- Our Top Team for Liposome siRNA-Encapsulated. Our company boasts a highly proficient liposome team comprised of seasoned scientists who possess robust theoretical expertise and extensive practical experience. This dedicated team focuses on the development of siRNA liposome products and has comprehensive experience across all stages, from initial development to final production.
Published Data
Technology: Liposomal siRNA Formulations for the Treatment of Herpes Simplex Virus-1 technique
Journal: Pharmaceutics
IF: 4.9
Published: 2022
Results: In this study, the authors identified a siRNA (siHSV) that inhibits gene expression of infected cell protein 0 (ICP0), which is important in regulating HSV infection. The selected siHSV is encapsulated in liposomes to overcome its poor stability, increase cell permeability, and extend the siRNA cycle time. Several siRNA targeting ICP0 have been designed and identified. The effects of various parameters, including formulation techniques, lipid composition, and proportion, were studied. The optimal liposomal siHSV formulation (Lip DOPE-siHSV) has ideal physicochemical properties, including nanometer size, low polydispersity index (PDI), neutral surface charge, high siHSV load, sphericity, high stability under in vitro physiological conditions, and long-term shelf life stability (>1 year, 4 °C). Liposomes exhibit deep internalization of human keratinocytes, are not cytotoxic in cell culture, have no harmful effects on mouse liver enzymes, and gradually escape from the lysosome. The therapeutic potential of siHSV liposomes was confirmed by plaque reduction tests and significant antiviral activity in 3D epidermal models, and the mechanism of action was validated by reducing ICP0 expression levels.
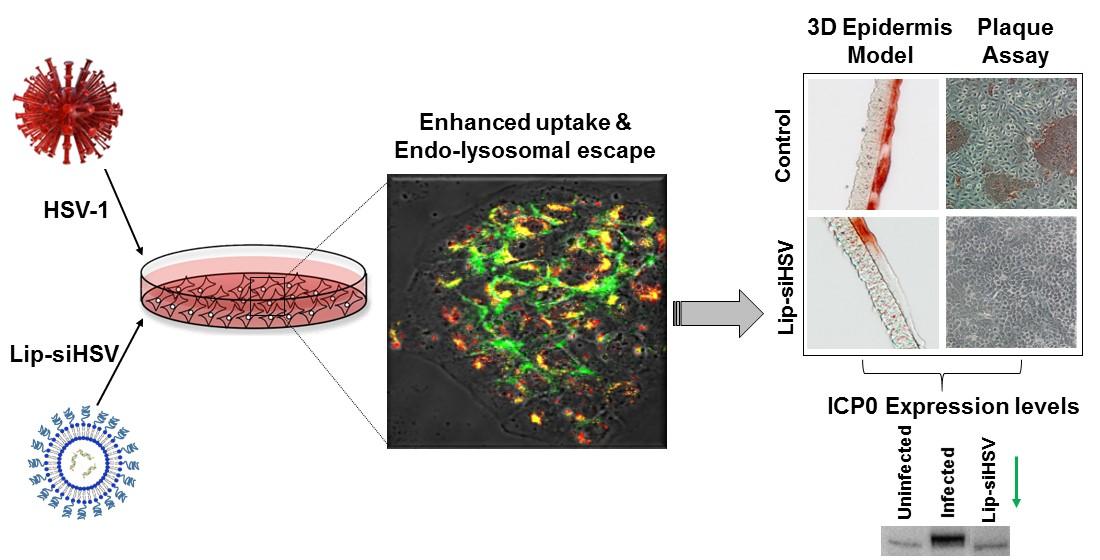 Fig.3 Illustration of liposomal siRNA formulations. (Jbara-Agbaria D, et al., 2022)
Fig.3 Illustration of liposomal siRNA formulations. (Jbara-Agbaria D, et al., 2022)
CD Formulation is committed to delivering high-quality liposome encapsulation services and advanced techniques for siRNA applications, tailored to the specific requirements of pharmaceutical companies and research institutions. Our expertise ensures reliable solutions that support innovative scientific research and therapeutic development. Contact us to learn how we can assist with your project needs.
References
- Carugo, Stefano., Sirtori, C., et al. Updates in Small Interfering RNA for the Treatment of Dyslipidemias. Current Atherosclerosis Reports. 2023. 25. 1-13. 10.1007.
- Yang F, Zheng Z, et al. SATB1 siRNA-encapsulated immunoliposomes conjugated with CD44 antibodies target and eliminate gastric cancer-initiating cells. Onco Targets Ther. 2018; 11: 6811-6825
- Jbara-Agbaria D, Blondzik S, et al. Liposomal siRNA Formulations for the Treatment of Herpes Simplex Virus-1: In Vitro Characterization of Physicochemical Properties and Activity, and In Vivo Biodistribution and Toxicity Studies. Pharmaceutics. 2022; 14(3): 633.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 Schematic illustration of siRNA. (Carugo, Stefano., et al, 2023)
Fig.1 Schematic illustration of siRNA. (Carugo, Stefano., et al, 2023) Fig.2 Liposome encapsulation for siRNA. (Yang F, et al., 2018)
Fig.2 Liposome encapsulation for siRNA. (Yang F, et al., 2018) Fig.3 Illustration of liposomal siRNA formulations. (Jbara-Agbaria D, et al., 2022)
Fig.3 Illustration of liposomal siRNA formulations. (Jbara-Agbaria D, et al., 2022)
