Liposome Encapsulation Service for Plasmid DNA
Inquiry
With the rapid advancements in cell and gene therapy, particularly in cancer and vaccine research, selecting an effective delivery system for DNA has become a crucial aspect of therapeutic development. DNA vaccines offer significant advantages, including high safety, ease of production, and cost-effectiveness, with promising applications in infectious diseases and cancer treatment. CD Formulation leverages advanced liposome encapsulation technology to develop stable plasmid DNA-encapsulated liposomes, tailored to meet the needs of researchers and pharmaceutical companies. Our innovative solutions aim to support the progress of cutting-edge DNA-based therapeutic research and development.
Advantages of Liposome Plasmid DNA Encapsulation
In comparison to earlier transfection protocols, liposomes and liposome-based complexes signify a substantial advancement in encapsulation technology, offering a promising foundation for the design of nanoparticles with transfection capabilities through the strategic incorporation of targeting elements.
- Improved antigen presentation. Liposomes can improve the stability and presentation of antigens to antigen-presenting cells (APCs).
- Controlled antigen release. Liposomes can release antigens slowly and controllably.
- Strong immune response. Liposomes as a vaccine carrier can effectively induce a strong immune response.
- Preventing enzymatic degradation. Liposomes can protect DNA from enzymatic degradation within cells.
- Enhanced cell uptake. Liposomes can enhance cell uptake, endosomal escape, and gene transfection.
- Fewer safety issues. Liposomes have fewer safety issues compared to other methods.
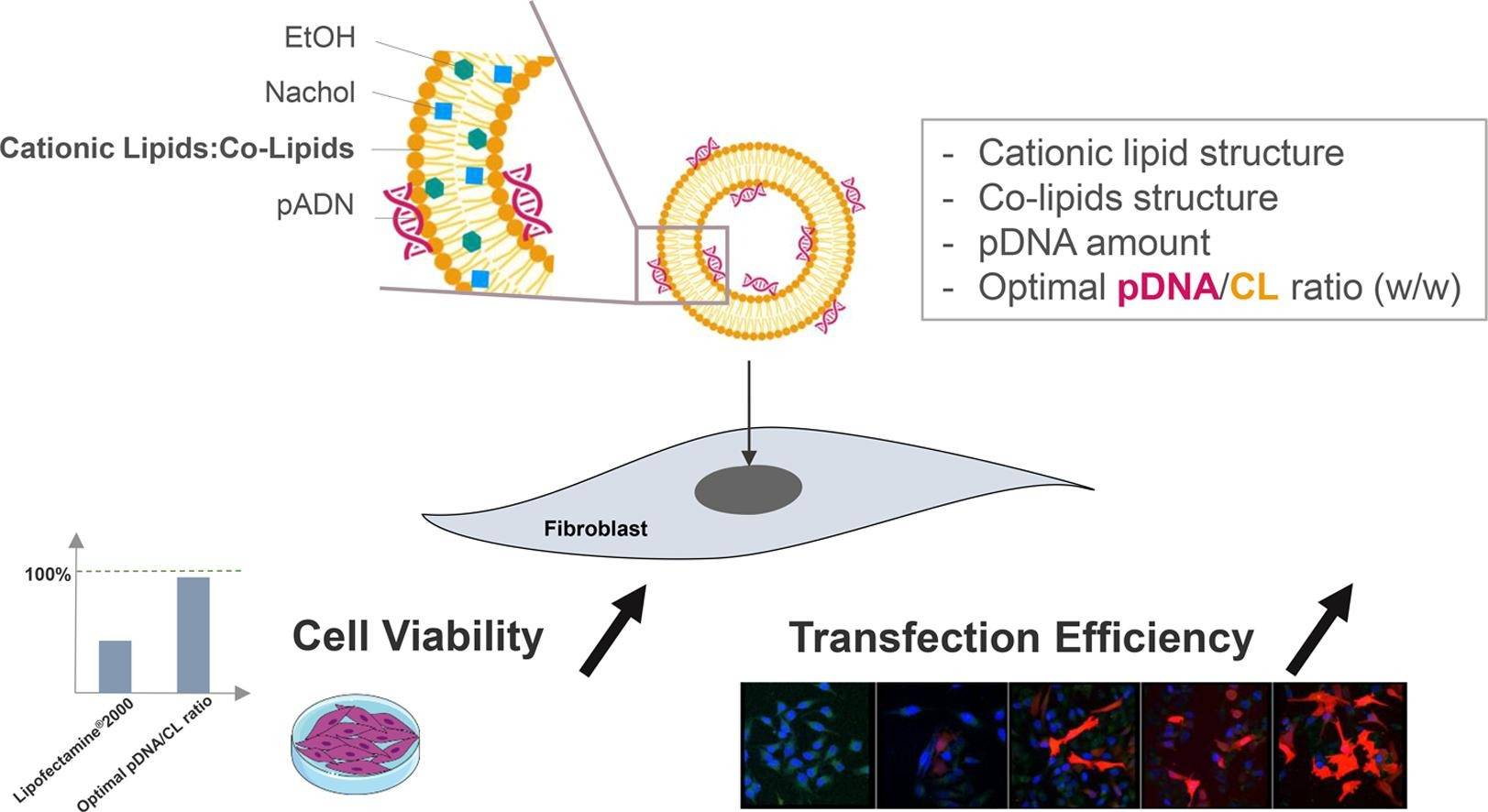 Fig.1 The illustration of liposome delivery for plasmid DNA. (Bellefroid C, et al, 2021)
Fig.1 The illustration of liposome delivery for plasmid DNA. (Bellefroid C, et al, 2021)
Our Liposome-Based Plasmid DNA Encapsulation Service
Plasmid selection and construction
The construction of plasmid vectors is a critical aspect of genetic engineering, essential for inserting target genes into plasmids. Its success directly impacts the progression of genetic research. Therefore, we will carefully control each step and validate every detail throughout the process.
Formulation of plasmid DNA-encapsulated liposomes
Our services range from formulation development studies in the small pilot phase of liposomes to process optimization. In the pilot phase, we conduct a variety of experiments to determine the optimal composition ratio and preparation conditions. This process includes not only the evaluation of different liposomal materials and their interactions, but also a systematic analysis of drug loading capacity, release properties, and stability. In addition, in process optimization, we will constantly adjust the parameters based on the preliminary experimental results to improve production efficiency and reduce costs.
In vitro transfection assays
The most important thing for liposome transfection is to prevent its toxicity. Therefore, the ratio of liposome to plasmid, cell density, length of transfection time, and serum content in the medium are all important issues that affect transfection efficiency. We explore suitable transfection conditions through experiments to improve the transfection efficiency.
Characterization service
We provide quality control services for liposomes. Test indicators include but not are limited to:
- Determination of content and examination of lipid-related degradation products
- Particle size and its distribution
- Encapsulation rate
- Surface charge
- In vitro drug release and drug leakage
- Structure and morphology
- Thermodynamic properties of lipid film, and shape of encapsulated drug
- pH
- Liposomal drug ratio
- Viscosity
- Sterility, pyrogen/bacterial endotoxin
Our Capacities for Liposome Plasmid DNA Encapsulation
| Techniques and Platforms |
Specifics |
Plasmid DNA Construction Platform
 |
- We cater to the requirements of research-grade, and other grade plasmid preparation services. We enable customers to efficiently execute cell transfection, DNA vaccine development, antibody production, cell therapy, gene therapy, and other research activities.
- Clients can select options flexibly based on their downstream applications.
|
Plasmid DNA Encapsulation Technology
 |
- Meeting the various research level.
- From prescription selection to process optimization, comprehensive performance analysis, and quality control, we strive to meet our customers' needs.
|
Characterization Platform
 |
- In vitro evaluation platform (such as cell uptake rate test, cytotoxicity test, release rate, and stability).
- In vivo evaluation platform (including cell targeting test, in vivo stability, and distribution).
- Physical & chemistry evaluation platform (size distribution, potential analysis, and encapsulation efficiency).
|
Why Choose CD Formulation?
Plasmid DNA Construction Platform
- A circular plasmid production platform and a linearized plasmid production platform that comply with GMP standards.
- Extensive experience in mature process development and GMP production.
- A comprehensive suite of services including plasmid construction, strain library establishment, process optimization, quality methodology research, stability studies, other grade plasmid production, as well as regulatory submission support.
A Premier Liposome Encapsulation Platform for Plasmid DNA
- The prepared liposomes exhibit good monodispersity, highly controllable particle size distribution, excellent batch-to-batch reproducibility, and high encapsulation efficiency.
- Low toxicity, reduced immunogenicity, and minimal cellular damage.
- Achieving transfection efficiencies exceeding 95%.
Top Teams
- Numerous interdisciplinary scientists including gene therapy, pharmaceutical preparation, and biology.
- Be proficient in the application of liposome -encapsulated plasmid DNA in medicine and vaccine.
- Proficient in the entire development process to production.
Published Data
Technology: Arginine-modified chitosan liposome systems technique
Journal: Colloids and Surfaces B: Biointerfaces
IF: 6.6
Published: 2020
Results: In this study, a chitosan-based carrier (chitosan carrier) was synthesized by a reverse evaporation method modified by arginine, and composed of chitosan, DOTAP/DOPE lipid complex. The structure of the chitosan carrier containing or not containing pDNA was analyzed by electrophoresis, zeta potential, dynamic light scattering, small-angle X-ray scattering, and isothermal titration calorimetry. The transfection efficiency and cytotoxicity were studied in HEK293 T cells. The results showed that the synthesized chitosan was a promising carrier for gene delivery.
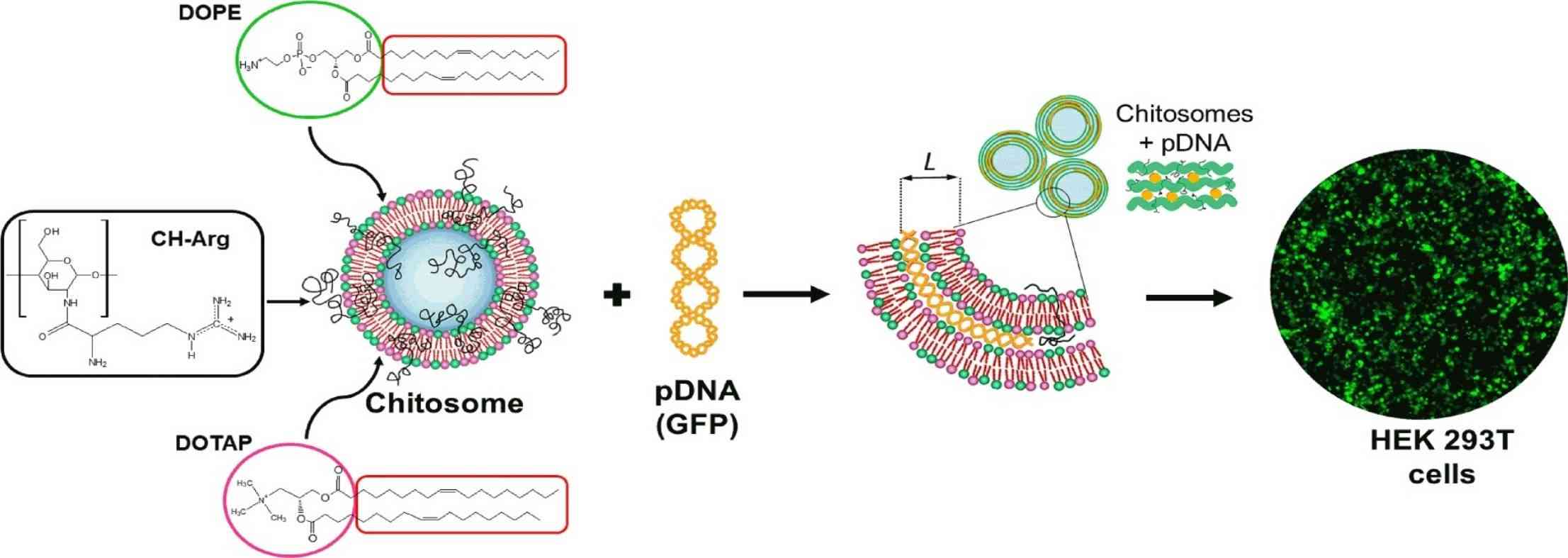 Fig.2 Arginine-modified chitosan complexed with liposome systems for plasmid DNA delivery. (Garcia BB, et al., 2020)
Fig.2 Arginine-modified chitosan complexed with liposome systems for plasmid DNA delivery. (Garcia BB, et al., 2020)
CD Formulation specializes in offering advanced liposome encapsulation technology for plasmid DNA, designed to meet the specific needs of pharmaceutical companies and researchers. Our innovative solutions ensure stability and efficiency, supporting cutting-edge research and development in gene therapy, vaccine development, and other DNA-based applications. Contact us to learn how we can assist with your scientific projects.
References
- Bellefroid C, Reusch C, et al. Systematic study of liposomes composition towards efficient delivery of plasmid DNA as potential application of dermal fibroblasts targeting. International Journal of Pharmaceutics. 2021 Jan 25; 593: 120122.
- Garcia BB, Mertins O, et al. Arginine-modified chitosan complexed with liposome systems for plasmid DNA delivery. Colloids and Surfaces B: Biointerfaces. 2020 Sep 1; 193: 111131.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 The illustration of liposome delivery for plasmid DNA. (Bellefroid C, et al, 2021)
Fig.1 The illustration of liposome delivery for plasmid DNA. (Bellefroid C, et al, 2021)


 Fig.2 Arginine-modified chitosan complexed with liposome systems for plasmid DNA delivery. (Garcia BB, et al., 2020)
Fig.2 Arginine-modified chitosan complexed with liposome systems for plasmid DNA delivery. (Garcia BB, et al., 2020)
