Liposome Encapsulation Service for Peptides
Inquiry
Liposomes serve as carriers for proteins and peptides. Liposomes can safeguard peptide structural integrity and biological activity, enhance stability, prolong half-life, and facilitate a sustained release. CD Formulation aims to utilize liposome encapsulation technology to develop stable peptide-encapsulated liposomes for pharmaceutical enterprises and researchers.
Importance of Liposome Peptide Encapsulation
Peptides typically consist of 10 to 100 amino acids with chains longer than 50 amino acids. They are usually referred to as proteins. Due to strong affinity and specificity, low toxicity, and the ability to regulate the protein interactions, peptides are playing an increasingly important role in the treatment of various diseases. However, peptides also have some problems, such as instability during processing and storage, poor permeability through non-injection routes, insufficient targeting at the site of action to maintain therapeutic effects, instability in biological fluids, and insufficient cell delivery capacity. One possible solution to these delivery challenges is to use liposome carrier systems.
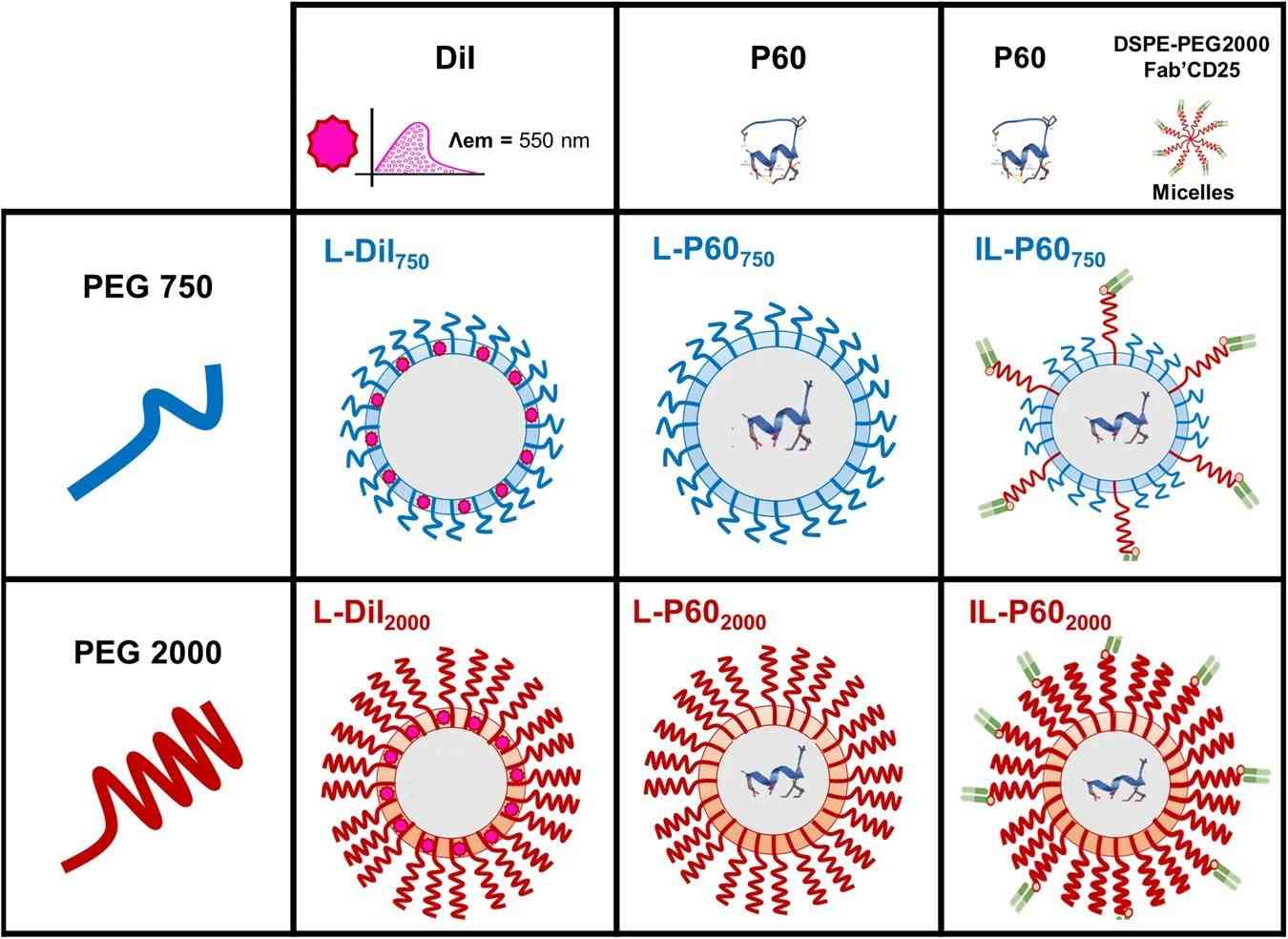 Fig.1 The illustration of liposome delivery for peptide. (Serrano, A., et al, 2024)
Fig.1 The illustration of liposome delivery for peptide. (Serrano, A., et al, 2024)
Our Liposome-Based Peptide Encapsulation Service
Formulation of peptide liposome encapsulation
We can enhance the stability of peptides and mitigate degradation during the drug delivery process through meticulous formulation development and optimization. Leveraging our universal formula, we are able to make flexible adjustments as needed. Furthermore, our formulation development emphasizes the tissue permeability of peptide formulations, thereby assisting our clients in diversifying administration routes for peptides (including extravascular, oral, nasal, ocular, and transdermal pathways).
Characterization service
We provide quality control services for liposomes. Analysis items include but not are limited to:
- Determination of content and examination of lipid-related degradation products
- Particle size and its distribution
- Encapsulation rate
- Surface charge
- In vitro drug release and drug leakage
- Structure and morphology
- Thermodynamic properties of lipid film, and shape of encapsulated drug
- Liposomal drug ratio
- Viscosity and pH
- Sterility, pyrogen/bacterial endotoxin
Our Capacities for Peptide Liposome Encapsulation
| Techniques and Platforms |
Specifics |
Peptide Encapsulation Technology
 |
- Freeze-drying technology. Advantageous for the formulation of thermosensitive peptide-based drug precursors within liposomes, although it incurs higher costs.
- Vacuum drying technology. Well-suited for lipid-soluble protein and peptide pharmaceuticals, exhibiting a relatively high encapsulation efficiency.
- CO2 supercritical technology. Addressing the limitations associated with organic solvent contact in conventional methods and ensuring preparation process at low temperatures.
|
Characterization Platform
 |
- Apparent characterization. Structure morphology and shape of encapsulated drug.
- In vivo & in vitroevaluation. Such as drug release and drug leakage.
- Physical & chemistry evaluation. Including size distribution, potential analysis, encapsulation efficiency, determination of content, and examination of lipid-related degradation products.
|
Why Choose CD Formulation?
A Premier Liposome Encapsulation Platform for Peptides
- This applies not only to small peptides but also to larger molecular peptides.
- Relevant application to healthcare products, cosmetics, pharmaceuticals, and research reagents.
- Appropriate for administration routes: intravenous, oral, transdermal, and intranasal methods.
Powerful Assessment System
- Outfitted with a range of sophisticated instruments for peptide and liposome characterization, covering the entire process of liposomes.
- Our proprietary pharmacokinetics platform precisely evaluates the absorption, distribution, metabolism, and excretion of liposomes.
- The quality research platform facilitates the assessment of physicochemical, in vivo, and in vitro indicators related to liposomes.
Our Highly Skilled and Exceptional Team of Professionals
- Having assisted numerous enterprises and academic institutions in successfully researching and developing liposome-encapsulated peptides.
- The technical team consists of multiple interdisciplinary scientists, spanning fields such as medicine, biological engineering, and materials science.
- All members of the research team are licensed professionals, particularly in vivo experiments who hold veterinary qualifications and relevant certificates.
Published Data
Technology: Foxp3 inhibitory peptide encapsulated in a novel CD25-targeted nanoliposome technique
Journal: Acta Pharmacol Sin
IF: 6.6
Published: 2024
Results: This study aimed to improve the stability and specific delivery of P60 by encapsulating it in liposomes targeting CD25, which is constitutively expressed in TreGs. P60 liposomes formulated with DSPE-PEG 750 or DSPE-PEG 2000 are incubated with DSPE-PEG 2000-Maleimide micelles coupled with anti-CD25 Fab' fragments to develop two targeting agents or immune liposomes (IL): IL-P60 2000 (only DSPE-PEG 2000) and IL-P60 750 (combined DSPE-PEG 750 and DSPE-PEG 2000). Notably, IL-P60750 inhibited human TREgs in in vitro trials, indicating that the formulation has transformational capabilities. In summary, IL-P60750 formulated with different PEG chain lengths showed anti-tumor efficacy by selectively inhibiting Treg activity and enhancing the anti-PD1 effect. Together, this novel IL represents a promising nanoplatform for cancer immunotherapy.
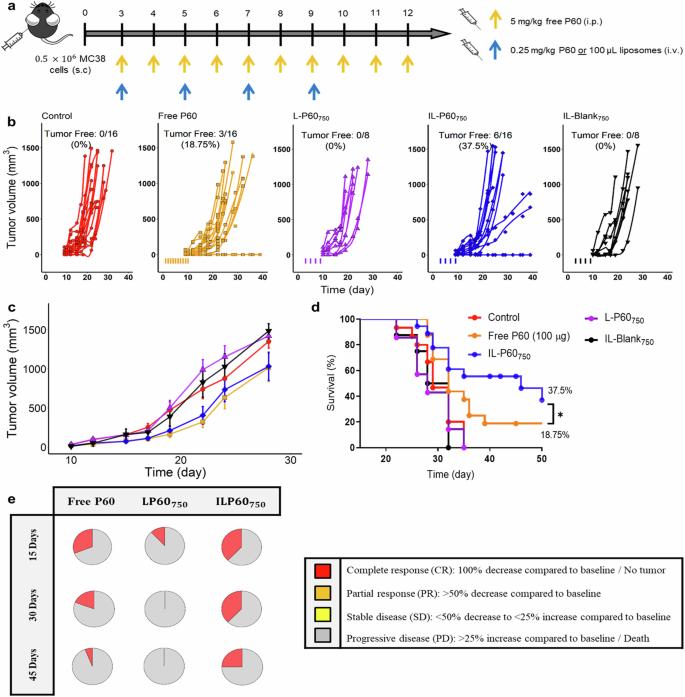 Fig.2 Antitumor in-vivo response assayed in MC38 tumor-bearing mice. (Serrano, A., et al, 2024)
Fig.2 Antitumor in-vivo response assayed in MC38 tumor-bearing mice. (Serrano, A., et al, 2024)
CD Formulation's team excels in providing innovative and efficient peptide liposome encapsulation techniques, tailored to meet the unique needs of pharmaceutical enterprises and researchers. Our advanced solutions are designed to enhance peptide stability and delivery, supporting a wide range of research and development projects. Contact us to discover how our expertise can contribute to your scientific advancements.
References
- Tretiakova, D.S., Vodovozova, E.L. Liposomes as Adjuvants and Vaccine Delivery Systems. Biochem. Moscow Suppl. Ser. A. 2022. 16, 1–20.
- Garcia BB, Mertins O, et al. Arginine-modified chitosan complexed with liposome systems for plasmid DNA delivery. Colloids and Surfaces B: Biointerfaces. 2020 Sep 1; 193: 111131.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 The illustration of liposome delivery for peptide. (Serrano, A., et al, 2024)
Fig.1 The illustration of liposome delivery for peptide. (Serrano, A., et al, 2024)

 Fig.2 Antitumor in-vivo response assayed in MC38 tumor-bearing mice. (Serrano, A., et al, 2024)
Fig.2 Antitumor in-vivo response assayed in MC38 tumor-bearing mice. (Serrano, A., et al, 2024)
