Liposome Encapsulation Service for mRNAs
Inquiry
Messenger RNA (mRNA) is the template for protein biosynthesis. It is increasingly recognized as a crucial active molecule in combating various diseases, including viral infections and cancer. CD Formulation specializes in assisting clients in developing stable mRNA liposomal formulations by our liposome technology.
About mRNAs
mRNA is a single-stranded nucleic acid composed of four nucleotides--uracil, guanine, adenine, and cytosine. It is first synthesized from a DNA template. After that, the coding information is transmitted to ribosomes for protein translation. The length of mRNA varies significantly; after transcription, each molecule undergoes editing, capping, and polyadenylation before being released into the cytosol.
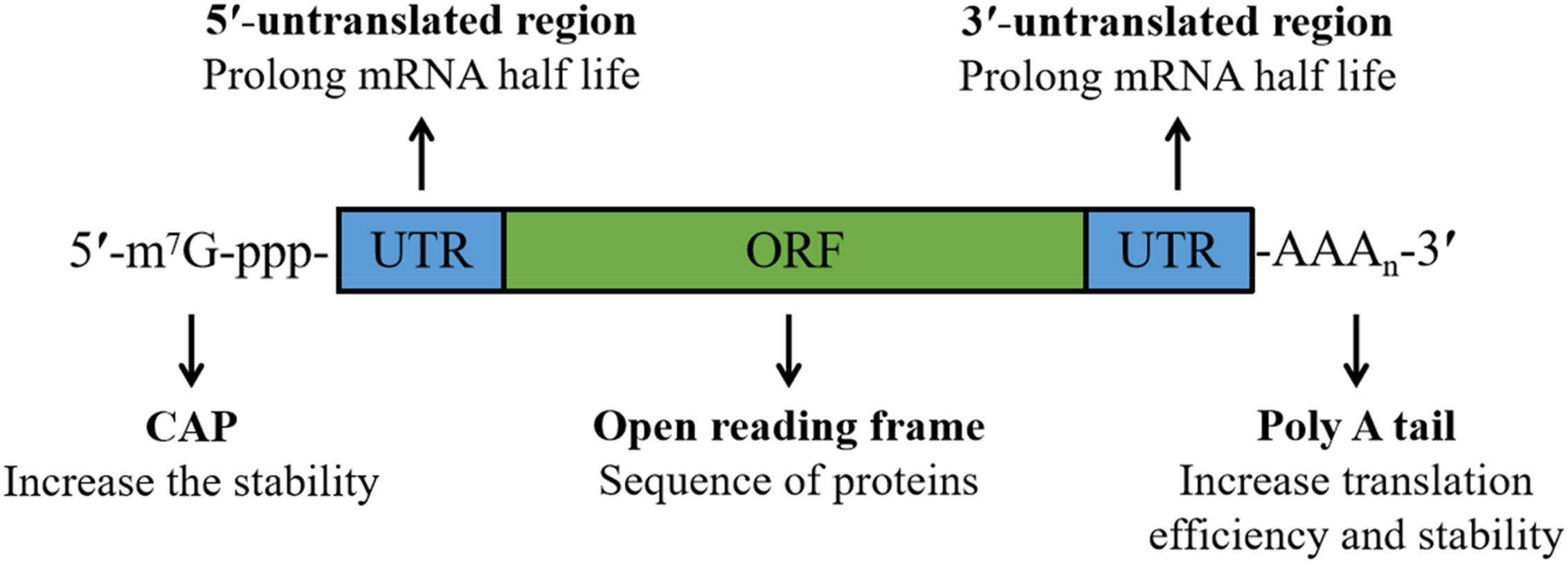 Fig.1 Schematic illustration of mRNA. (Zhang W, et al, 2023)
Fig.1 Schematic illustration of mRNA. (Zhang W, et al, 2023)
Importance of Liposome mRNA Encapsulation
- Prevent Degradation. Liposomes can protect mRNA from enzymatic degradation.
- Endocytosis and Escape from Endosomes. Positively charged liposomes can electrostatically wrap negatively charged mRNA, helping the delivery system bind to the cell membrane. Then, mRNA can be endocytosed into the cytosol. However, it must escape from endosomes and liposomal carriers to reach the cytosol and undergo transcription. Liposomes can fuse with the endosomal membrane through the phospholipid bilayer, helping mRNA escape.
- High Encapsulation Efficiency. Since the positively charged components of liposomes can be complex with more negatively charged mRNA, surface-positively charged liposomal formulations can achieve higher encapsulation efficiency.
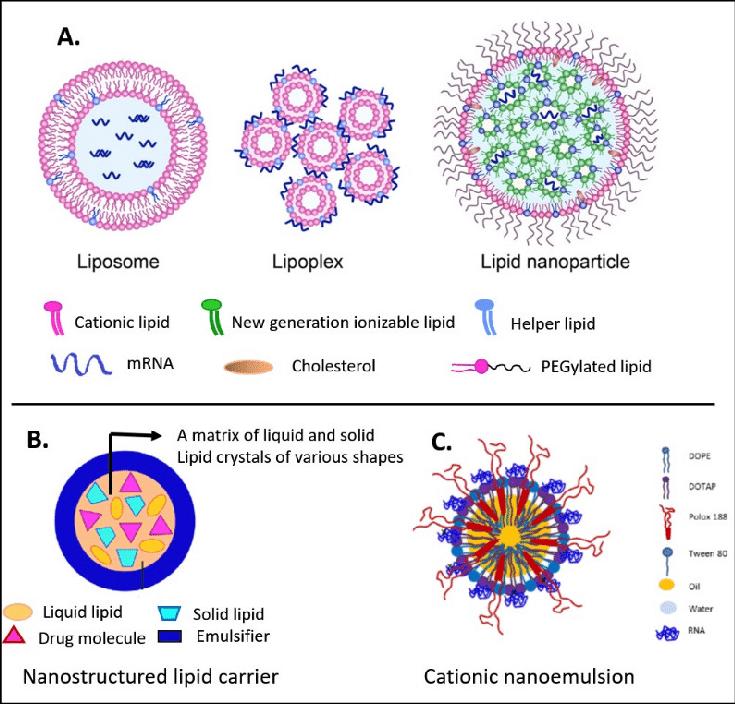 Fig.2 Key lipid nanocarriers of mRNA: (A) liposome, lipoplex, and lipid nanoparticle; (B) nanostructured lipid carrier; (C) cationic nanoemulsion. (Aldosari, Basmah., et al., 2021)
Fig.2 Key lipid nanocarriers of mRNA: (A) liposome, lipoplex, and lipid nanoparticle; (B) nanostructured lipid carrier; (C) cationic nanoemulsion. (Aldosari, Basmah., et al., 2021)
Our Liposome-Based mRNA Encapsulation Service
Lipid selection and formulation design
We enhance the stability of liposomes through the systematic screening of lipid formulations. Lipids such as DOTAP can interact directly with cell surfaces via electrostatic interactions, facilitating their uptake by dendritic cells (DC) and subsequently promoting antigen presentation to bolster cellular immune responses. Furthermore, incorporating cholesterol into the DOTAP formulation can significantly improve liposome stability, while polyethylene glycol (PEG) serves to mitigate modulation effects and nonspecific uptake, thereby prolonging their circulation half-life in vivo. In certain instances, specifically synthesized cationic lipids that possess ionizable properties are required for various types of liposomes.
Liposome type selection
Our mRNA liposome delivery system can be classified into three categories: cationic, ionizable, and others. Among these, 1,2-di-O-octadecenyl-3-trimethylammonium propane (DOTMA) and dioleoyl-3-trimethylammonium propane (DOTAP) are utilized either independently or in conjunction with other materials in mRNA delivery systems. Additionally, our ionizable liposomes also assist our clients in studies related to cancer immunity and gene editing.
Characterization service
Our characterization of mRNA liposomes encompasses assessments of particle size, in vitro delivery, in vivo delivery, stability, and encapsulation efficiency.
Our Capabilities for Liposome mRNA Encapsulation
| Techniques and Platforms |
Specifics |
| mRNA encapsulation technology |
- Lipid formulation development
- Lipid synthesis and modification
- Liposome type and methods screening
|
| Characterization platform |
- In vitro evaluation techniques and in vivo evaluation techniques
- Physical and chemistry evaluation
- A wide range of characterization tools and instruments
|
Why Choose CD Formulation?
- Excellent Liposome mRNA Encapsulation Techniques. Our cutting-edge mRNA liposome encapsulation technology significantly improves the bioavailability and targeted delivery of various mRNAs. Depending on the intended delivery target and location, we adeptly modify the composition and size of the liposomes by leveraging the unique characteristics of each mRNA. Furthermore, through advanced surface modification techniques, we enhance the affinity of these liposomes for specific cell types or tissues, thereby facilitating advancements in precision medicine.
- Exclusive Analytical Platform for Liposomes with mRNAs. Our advanced mRNA liposome analytical system is meticulously engineered to comply with pharmaceutical development standards. This system seamlessly integrates a range of specialized instruments and software tools for liposome characterization, providing comprehensive data support that empowers researchers to thoroughly investigate the structural characteristics, physical and chemical properties, and potential applications of liposomes in mRNA delivery.
- Our Top Team. Our company has a team of highly proficient technical experts, consisting of seasoned scientists with strong theoretical knowledge and hands-on experience. This diverse group focuses on biochemistry, drug development, and materials science, conducting in-depth research at every stage of small molecule liposome product creation. Our team meticulously designs experiments and interprets data to enhance formulations while boosting stability, biocompatibility, and release efficiency.
Published Data
Technology: Intranasal delivery of cationic liposome-protamine complex mRNA technique
Journal: Cellular Immunology
IF: 3.7
Published: 2020
Results: In this study, the authors investigated the methodology for administering mRNA through the nasal cavity, utilizing positively charged bovine prolactin to enhance mRNA concentration and form stable polycation-mRNA complexes, which were subsequently encapsulated in DOTAP/Chol/DSPE-PEG cationic liposomes. The LPC/bovine prolactin complex demonstrated superior vaccine particle uptake efficiency and a heightened capacity to stimulate dendritic cell maturation in vitro, thereby further eliciting a robust anti-tumor immune response. Intranasal delivery of mRNA encoding cytokeratin 19 to mice via cationic LPC resulted in a pronounced cellular immune response within an aggressive Lewis lung cancer model and impeded tumor growth. In this study, the authors substantiate that cationic LPC can serve as a safe and effective adjuvant. However, this mRNA formulation lays the groundwork for human anti-cancer vaccine administration.
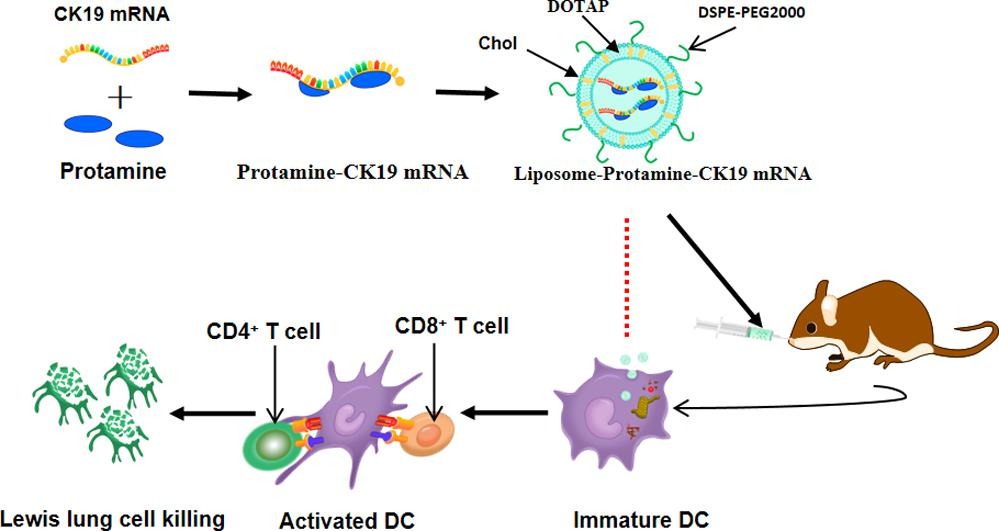 Fig.3 The mechanism of Intranasal delivery of cationic liposome-protamine complex mRNA vaccine. (Mai Y, et al., 2020)
Fig.3 The mechanism of Intranasal delivery of cationic liposome-protamine complex mRNA vaccine. (Mai Y, et al., 2020)
CD Formulation provides excellent mRNA liposome encapsulation services and technology for researchers. Our expertise ensures reliable and innovative solutions to support your scientific and development goals. Contact us to discuss how we can assist with your projects.
References
- Zhang W, Jiang Y, et al. Lipid carriers for mRNA delivery. Acta Pharmaceutica Sinica B. 2023 Oct 1; 13(10): 4105-26.
- Aldosari, Basmah., Alfagih, Iman., et al. Lipid Nanoparticles as Delivery Systems for RNA-Based Vaccines. Pharmaceutics. 2021. 13. 206. 10.3390.
- Mai Y, Guo J, et al. Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cellular Immunology. 2020 Aug 1; 354: 104143.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Schematic illustration of mRNA. (Zhang W, et al, 2023)
Fig.1 Schematic illustration of mRNA. (Zhang W, et al, 2023) Fig.2 Key lipid nanocarriers of mRNA: (A) liposome, lipoplex, and lipid nanoparticle; (B) nanostructured lipid carrier; (C) cationic nanoemulsion. (Aldosari, Basmah., et al., 2021)
Fig.2 Key lipid nanocarriers of mRNA: (A) liposome, lipoplex, and lipid nanoparticle; (B) nanostructured lipid carrier; (C) cationic nanoemulsion. (Aldosari, Basmah., et al., 2021) Fig.3 The mechanism of Intranasal delivery of cationic liposome-protamine complex mRNA vaccine. (Mai Y, et al., 2020)
Fig.3 The mechanism of Intranasal delivery of cationic liposome-protamine complex mRNA vaccine. (Mai Y, et al., 2020)
