Liposome Delivery - CD Formulation
Call Us:
- Home
- Services
- Liposome Formulation Development
- Liposome Formulation Process Development
- Liposome Analysis and Characterization
- Liposome Biological Evaluation
- Universal Liposome Characterization
- Liposome Size Distribution Testing
- Liposome Zeta Potential Testing
- Encapsulation Efficiency (EE%) Testing
- Liposome Drug Loading Testing
- Thermodynamic Properties Analysis of Liposome Membranes
- Liposome Morphology/Structure Analysis
- Liposome Membrane Permeability Testing
- Liposome Lamellarity Analysis
- Liposome Phase Transition Temperature Analysis
- Oral Liposome Characterization
- Transdermal Liposome Characterization
- Injectable Liposome Characterization
- Liposome Aerosol Characterization
- Lyophilized Liposome Characterization
- Ocular Liposome Characterization
- Liposome Customization Services
- Custom Long-circulating Liposome Service
- Custom Proliposome Service
- Custom Polymer-modified Liposome Service
- Custom Environmentally Responsive Liposome Service
- Custom pH-sensitive Liposome Service
- Custom Temperature Responsive Liposome Service
- Custom Light-responsive Liposome Service
- Custom Magnetically Responsive Liposome Service
- Custom Ultrasound Responsive Liposome Service
- Custom Dual Responsive Liposome Service
- Custom Enzyme Responsive Liposome Service
- Custom Immunoliposome Service
- Custom Glucose Modified Liposome (GML) Service
- Custom Flexible Liposome Service
- Custom GSH-liposome Service
- Custom Reactive Oxygen Species (ROS)-responsive Liposome Service
- Custom Fusogenic Liposome Service
- Custom Multivesicular Liposome Service
- Custom Hollow Fiber Liposome Service
- Custom Labeled Liposome Service
- Liposome Encapsulation Services
- Liposome Encapsulation Service for Small Molecules
- Liposome Encapsulation Service for mRNAs
- Liposome Encapsulation Service for siRNAs
- Liposome Encapsulation Service for microRNAs
- Liposome Encapsulation Service for Plasmid DNA
- Liposome Encapsulation Service for Peptides
- Liposome Vaccine Encapsulation Service
- Liposome Drug Delivery System Development
- Liposome Quality Control and Testing
- Liposome cGMP Manufacturing
- Technologies
- Applications
- Liposome Formulation for Cancer Treatment Research
- Liposome Formulation for Parasitic Diseases and Infections Research
- Liposome Formulation for Tissue Engineering Research
- Liposome Formulation for Cosmetics Industry
- Liposome Formulation for Food Industry
- Liposome Formulation for Agricultural Research
- Liposome Formulation for Gene Therapeutic Research
- Order Online
- Company
- Inquiry


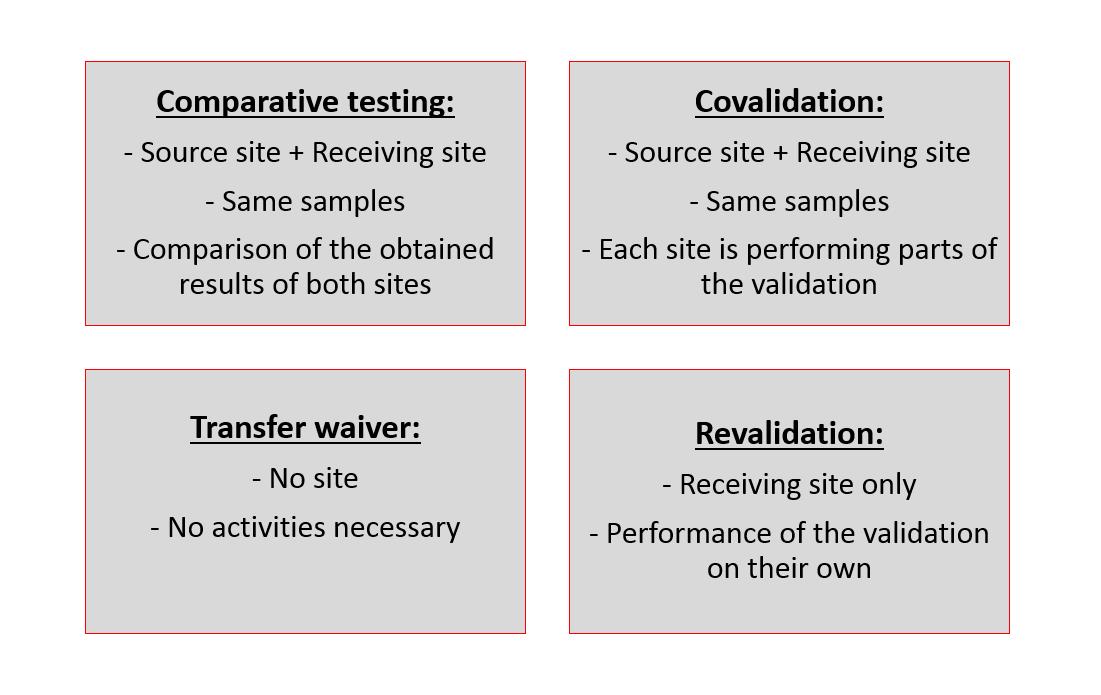 Fig.1 Liposome method transfer approaches. (Janet Thode, 2018)
Fig.1 Liposome method transfer approaches. (Janet Thode, 2018)
