Liposome Analytical Method Development and Validation
Inquiry
Liposomes serve as carriers in commercial pharmaceutical formulations (Pharmaceutical Formulations, PFs) and dietary supplements (Dietary Supplements, DSs), effectively safeguarding active ingredients from degradation while prolonging the half-life of injectable drugs. Despite the rapid commercialization of liposome products, it is imperative to establish a systematic approach to characterizing their physical and chemical properties. CD Formulation aims to assist pharmaceutical enterprises and institutions in developing and validating advanced liposome analytical methods.
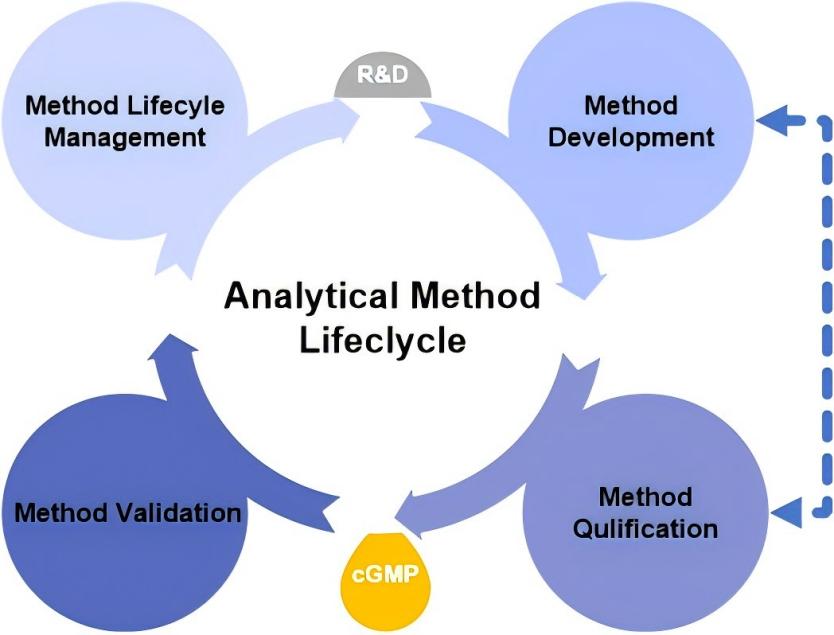 Fig.1 Liposome analytical method development and validation. (CD Formulation)
Fig.1 Liposome analytical method development and validation. (CD Formulation)
Importance of Liposome Analytical Method Development and Validation
The development and validation of analytical methods represent ongoing, interrelated activities throughout the drug development continuum. Validation practices confirm whether a specific method accurately measures intended parameters and delineates the performance limits of such measurements. Although it may appear paradoxical, validated methods yield results within a defined range of uncertainty. These outcomes are vital for advancing drug development, as they establish the foundational knowledge base that underpins the product's evolution. Investing of time and resources in creating scientifically robust, transferable analytical methods should align with the respective stage of drug development. Furthermore, resource allocation for method validation must be continuously calibrated against regulatory requirements and the practicalities associated with product commercialization.
Our Liposome Analytical Method Development and Validation
Method Development
Before the development phase, we need to gather comprehensive information regarding the properties of the liposome formulation components. This data will facilitate the selection of appropriate parameters for analytical methods and guide the choice of suitable analytical techniques based on these properties. During development, the method selection process typically necessitates referencing legal standards alongside other scholarly publications such as pharmacopoeias and national food safety standards from various countries.
Method Optimization
Once an analytical method is established, it must be optimized for specific applications and analysis stages. After the preliminary screening, our laboratory engages in an extensive method optimization process that emphasizes critical parameters including gradient, detection wavelength, flow rate, column type, column temperature, sample concentration, and injection volume. Concurrently, we can perform both single-factor and multi-factor optimizations to preliminarily identify optimal analytical conditions, significantly enhancing the efficiency of the method optimization process.
Preliminary Validation
Following the initial selection of the analytical method, it is imperative to validate or prevalidate the method, which encompasses specific attributes such as forced degradation studies, limits of detection (LOD) and quantification (LOQ), method robustness, accuracy, and precision, among others. If deemed necessary, further optimization of the method may be conducted to establish the final chromatographic conditions.
Full Validation
Our validation of analytical methods for quality standards aims to demonstrate that the selected method is suitable for the specific detection requirements, encompassing the physical state, identification, inspection, and quantification of the active pharmaceutical ingredient and its formulations. The detection parameters we offer include identification, impurity assessment (limit tests and quantitative analyses), as well as quantitative determinations (content analysis, dissolution testing, release rate evaluation, etc.). The scope of method validation encompasses specificity, linearity, range, accuracy, precision, detection limit, quantitation limit, and robustness.
Method Verification
This service aims to demonstrate that the validated methods fulfill the requirements for product testing, while also clarifying the scope, including inspection methods that do not necessitate validation for materials and products, as well as pharmacopoeial methods and other legally validated standards. It is crucial to pay attention to the validation of method transfer between different laboratories. This is substantiated through method validation to confirm its appropriateness under laboratory conditions.
Our Capacities for Liposome Analytical Method Development and Validation
| Techniques and Platforms |
Specifics |
| Liposome Analytical Method Development Platform |
- Study of properties of objects to be measured
- Screening of detection means and standard
- Screening of pre-treatment methods
- Analytical methodology development (accuracy, precision, specificity, detection limits, linearity, range, and durability)
- Chemical sample analysis and biological sample (metabolite, body fluid sample) analysis
- Analysis of metabolites of phospholipids and cholesterol
|
| Instruments & Facility |
- HPLC-MS/MS; GC-MS; HPLC-UV; HPLC-ELSD
- Optical microscope
- Scanning electron microscope
- In vitro models for drug dynamic dissolution
|
Why Choose CD Formulation?
Expertise in Liposome Analytical Method Development and Validation
- Proficient in liposome methodology development.
- Support the development of analytical methods for liposome products throughout the development process, including method establishment, method validation, method transfer, and method verification.
A Powerful Analysis Platform
- A range of sophisticated instruments for liposome physical and chemical characterization.
- The quality research platform is specifically designed for liposome drugs to efficiently evaluate the characteristics of related liposomes in vivo and in vitro.
A team of Highly Skilled and Esteemed Professionals
- We have effectively supported numerous enterprises and academic institutions in the liposome analytical method development.
- Our technical team is comprised of a diverse array of scientists who are experts in analytical chemistry and lipid-based drug delivery.
Published Data
Technology: an analytical technology utilizing multi-detector asymmetric flow field fractionation
Journal: Journal of Controlled Release.
IF: 4.5
Published: 2022
Results: In this study, the authors developed a method utilizing multi-detector asymmetric flow field fractionation (MD-AF4) and validated for the optimization of separation and characterization of liposome drug formulations into their constituent populations, with a focus on accuracy and reproducibility, irrespective of whether the properties exhibit mono- or multi-peaked distributions. Furthermore, the findings indicate that this method is adept at quantifying liposomes in the presence of serum proteins while providing insights into both structural attributes and physical stability of the formulation. This approach addresses critical needs identified in regulatory science and applies to novel therapeutic entities (NEPs) such as Doxil; with certain modifications, it can also be extended to assess other forms of NEPs, including lipid nanoparticle therapies. Overall, this methodology will expedite NEP development and streamline regulatory review processes, thereby facilitating a more rapid transition from laboratory research to clinical application. Additionally, the technique employed herein—stemming from international collaboration among leading non-regulatory organizations—can be replicated to bridge existing gaps encountered during analyses of various NEP types.
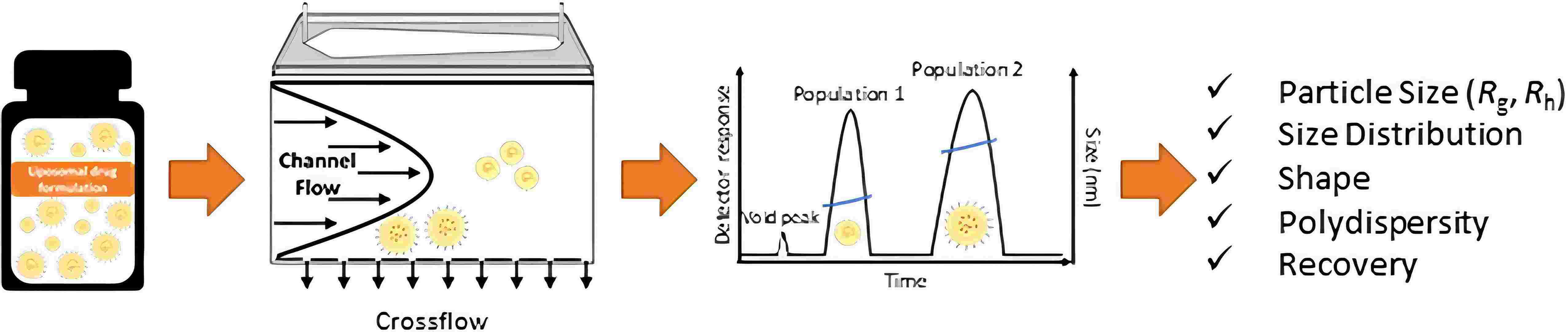 Fig.3 Illustration of analytical method for liposomes. (Parot J, et al, 2020)
Fig.3 Illustration of analytical method for liposomes. (Parot J, et al, 2020)
CD Formulation specializes in liposome analytical method development, supporting researchers and pharmaceutical development projects. Please do not hesitate to contact us if you require any assistance.
References
- Parot J, Caputo F, et al. Physical characterization of liposomal drug formulations using multi-detector asymmetrical-flow field flow fractionation. Journal of Controlled Release. 2020 Apr 10; 320: 495-510.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Liposome analytical method development and validation. (CD Formulation)
Fig.1 Liposome analytical method development and validation. (CD Formulation) Fig.3 Illustration of analytical method for liposomes. (Parot J, et al, 2020)
Fig.3 Illustration of analytical method for liposomes. (Parot J, et al, 2020)
