Injectable Liposome Particulate Matter Analysis
Inquiry
Particulate matter can cause safety concerns and is one of the key quality attributes (CQA) of injections. In general, the particles in injections are divided into exogenous particles, endogenous particles, and intrinsic particles. CD Formulation has a powerful platform for particle characterization and analysis of injected liposomes, covering particle analysis instruments ranging from nanoscale, and sub-micron to micron. We can provide comprehensive particle size characterization and distribution testing services, particle morphology characterization services, and identification of insoluble particles and foreign bodies, etc. We can determine particle size distribution and develop and verify particle size distribution methods according to USP <429> and other pharmacopeia methods to help customers establish particle size control quality standards.
Why Conduct Analysis of Injectable Liposome Particulate Matter?
The quality of injectable liposomes is related to the content of active ingredients, sterility, related substances, and foreign bodies, and the strict control of these indexes is related to the effectiveness and safety of injection. Among them, foreign bodies are divided into insoluble particles and visible foreign bodies. The requirements in pharmacopeia for "Particulate Matter in Injections" are divided into visible and sub-visible particles, and they prescribe that control is performed in completely different ways for those two categories. Insoluble particles in injections refer to movable insoluble particles other than bubbles that are unintentionally present in the solution. Once insoluble particles enter the body with the injections, they are capable of being carried by the blood, but cannot undergo metabolism, which may cause difficult-to-detect and potentially serious harm to the human body, such as inflammation, vascular injury, granuloma, vascular embolism, pyrogen reaction, allergic reaction, tumor or tumor-like reaction and other adverse reactions. Therefore, foreign bodies need to be strictly controlled.
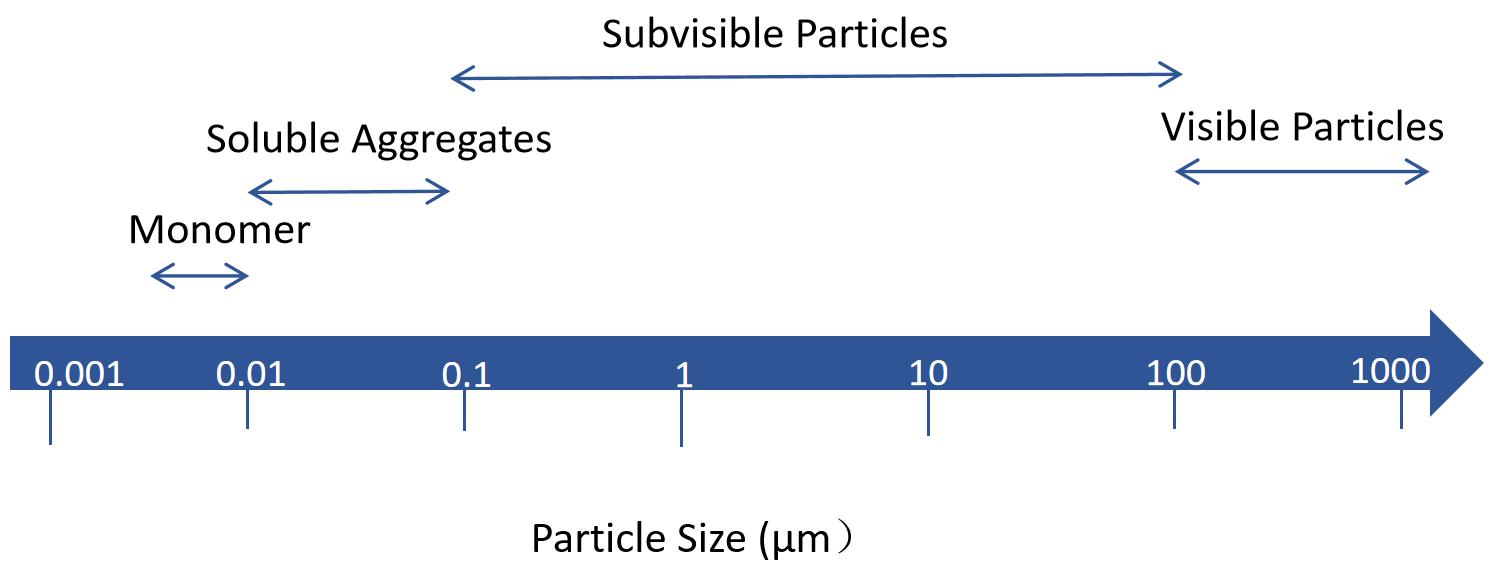 Fig.1 Visible and subvisible particles in injection. (CD Formulation)
Fig.1 Visible and subvisible particles in injection. (CD Formulation)
Our Services for Injectable Liposome Particulate Matter Analysis
Non-destructive Test
When foreign particles are detected, the initial step involves conducting a particle-free inspection. This crucial step primarily focuses on analyzing particle attributes, encompassing size, quantity, shape (e.g. fiber, spherical, and non-spherical), color, type (e.g., metal or glass shards), and sedimentation behavior to ensure accurate particle identification. We provide various methods such as visual inspection and microscopic techniques.
Particle Destructive Test
When the initial step of particle damage-free detection fails to provide comprehensive particle information, additional analytical characterization tools are required to further ascertain the composition and origin of the particles. The subsequent step involves particle damage detection, which is opening the sealed container and separating the particles. Throughout the separation process and sample handling, utmost care must be exercised to prevent false positive results caused by delicate particles such as oxidation, fracture, and dissolution, as well as avoid introducing extraneous substances like environmental particles. Furthermore, it is crucial to ensure an adequate volume of samples containing particles. Following separation, methods for analyzing the components of the particles include infrared spectroscopy (FTIR), Raman spectroscopy, and time-of-flight secondary ion mass spectrometry (TOF-SIMS).
Treated Particle Destructive Test
In the second step of particulate damage detection, if the isolated particles cannot undergo further processing beyond separation and membrane filtration, the third step involves conducting common biochemical tests on the destructive particles. The technologies we offer include matrix-assisted laser desorption/ionization (MALDI), capillary gel electrophoresis (CE-SDS), SDS polyacrylamide gel electrophoresis (SDS-PAGE), and peptide mapping analysis, among others.
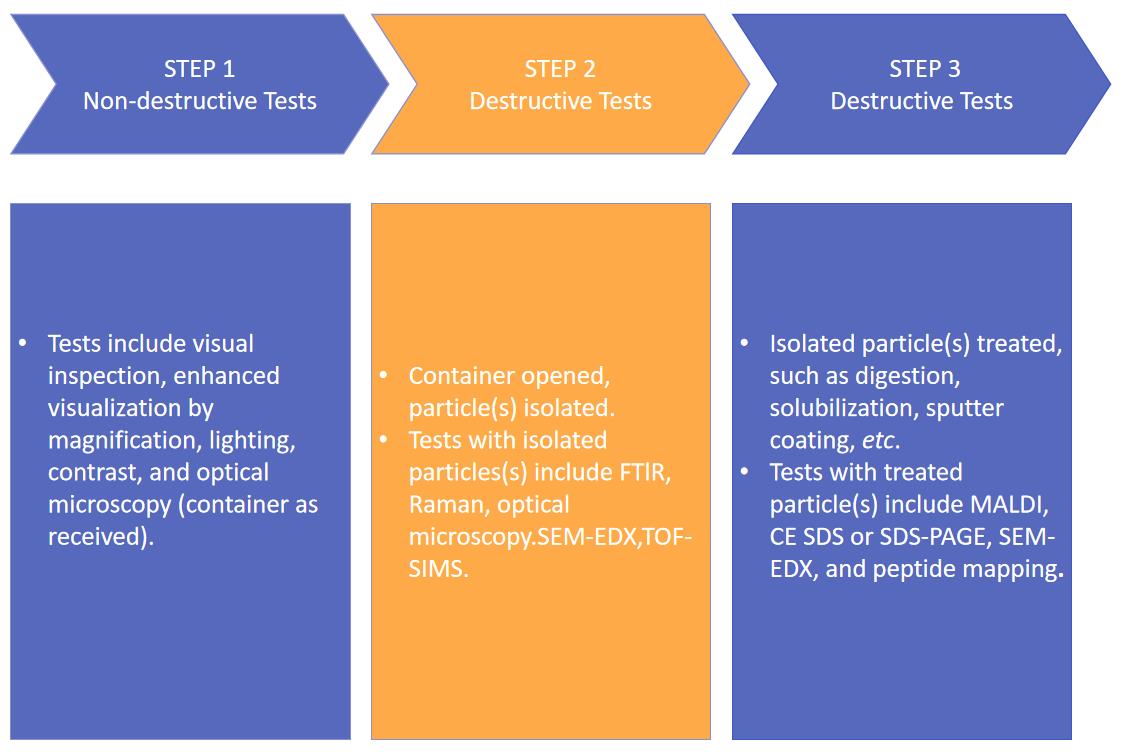 Fig.2 Our workflow of injectable liposome particulate matter analysis. (CD Formulation)
Fig.2 Our workflow of injectable liposome particulate matter analysis. (CD Formulation)
Our Platforms for Injectable Liposome Particulate Matter Analysis
| Items |
Detailed Information |
| Non-destructive Test |
- Focuses on analyzing particle attributes, encompassing size, quantity, shape (such as fiber, spherical, and non-spherical), color, type (e.g., metal or glass shards), and sedimentation behavior to identify the particles.
- We provide various methods such as visual inspection and microscopic techniques.
|
| Particle Destructive Test |
- Open the sealed container and separate the particles.
- Great care must be taken throughout the separation process and sample handling to prevent false positive results caused by fine particles such as oxidation, breakage, and dissolution, as well as to avoid the ingress of foreign substances such as environmental particles.
- Analysis techniques include infrared spectroscopy (FTIR), Raman spectroscopy, and time-of-flight secondary ion mass spectrometry (TOF-SIMS).
|
| Treated Particle Destructive Test |
- This platform involves conducting common biochemical tests on the destructive particles.
- The technologies we offer include matrix-assisted laser desorption/ionization (MALDI), capillary gel electrophoresis (CE-SDS), SDS polyacrylamide gel electrophoresis (SDS-PAGE), and peptide mapping analysis, among others.
|
Our Key Advantages in Injectable Liposome Particulate Matter Analysis
- Strictness. The team strictly follows pharmacopeia standards and industry guidelines. We have established multiple strict lab procedures. For injectable liposome particulate matter analysis, we can perform the analysis according to the national pharmacopeia (USP/Ph. Eur./JP) such as USP <1788>, USP <788>, USP <787>, etc.
- Extensive and sophisticated. We can detect particles ranging from <0.001 μm to > 3,000 μm, using various techniques to develop and validate particle size methods.
- Cost-effective. We provide systematic experimental solutions for customers to solve their problems comprehensively.
Published Data
Technology: Background membrane imaging versus flow technologies in subvisible particle analysis.
Journal: International Journal of Pharmaceutics.
IF: 5.3
Published: 2020
Results: HORIZON, a recently developed high-throughput background membrane imaging (BMI) technology, was evaluated to determine its ability to quantify subvisible particles (SVP) generated during protein therapy development. The HORIZON platform method is optimized and compared with three distinct SVP counting techniques. We found that the accuracy of BMI was similar to all other techniques. The concentration and size data generated by BMI closely match current flow imaging techniques while reducing the time, cost, and sample requirements of SVP quantization.
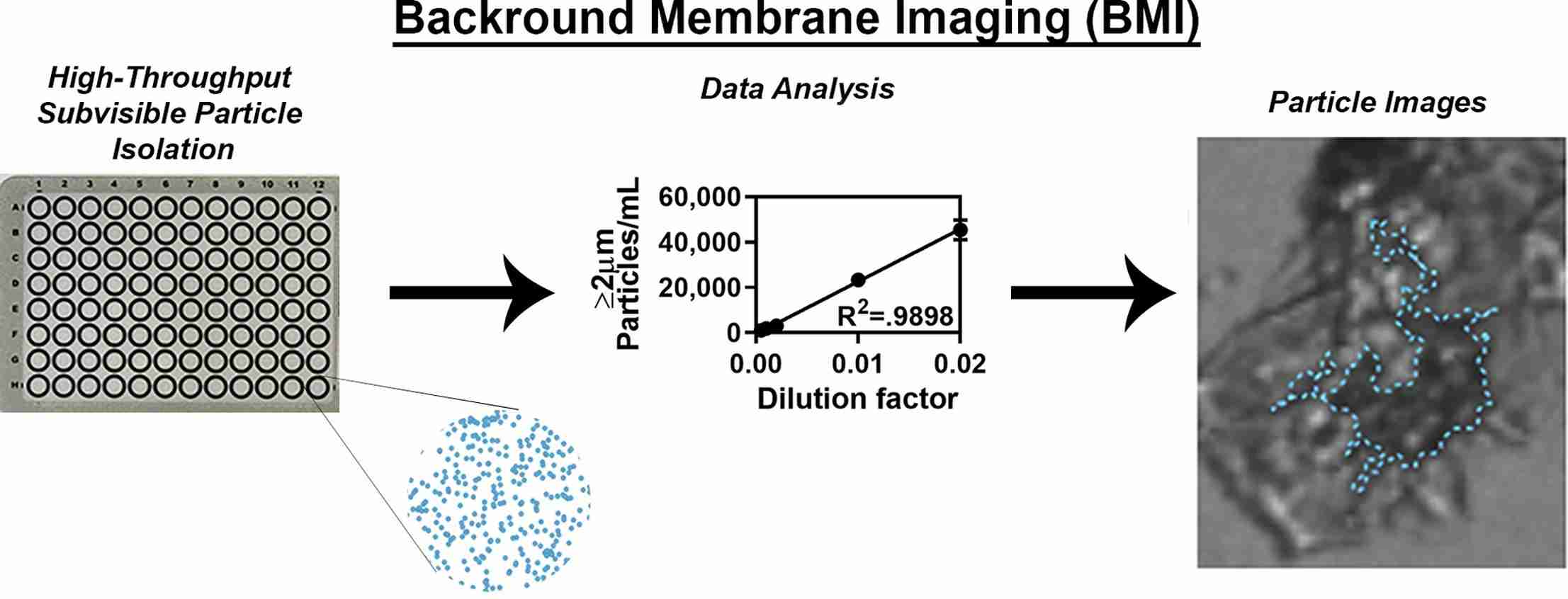 Fig.3 The schematic diagram of background membrane imaging versus flow technologies for subvisible particle analysis. (Stephanie K. Vargas, et al., 2020)
Fig.3 The schematic diagram of background membrane imaging versus flow technologies for subvisible particle analysis. (Stephanie K. Vargas, et al., 2020)
Based on our platforms for injectable liposome particulate matter analysis, CD Formulation has established a complete program of particulate matter analysis. If you need any kind of assistance, please contact us immediately.
References
- Stephanie K. Vargas, Aydin Eskafi, et al. A comparison of background membrane imaging versus flow technologies for subvisible particle analysis of biologics. International Journal of Pharmaceutics. 2020. Volume 578. 119072.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Visible and subvisible particles in injection. (CD Formulation)
Fig.1 Visible and subvisible particles in injection. (CD Formulation) Fig.2 Our workflow of injectable liposome particulate matter analysis. (CD Formulation)
Fig.2 Our workflow of injectable liposome particulate matter analysis. (CD Formulation) Fig.3 The schematic diagram of background membrane imaging versus flow technologies for subvisible particle analysis. (Stephanie K. Vargas, et al., 2020)
Fig.3 The schematic diagram of background membrane imaging versus flow technologies for subvisible particle analysis. (Stephanie K. Vargas, et al., 2020)
