Injectable Liposome Formulation Development
Inquiry
CD Formulation has been dedicated to the research of long-acting injectable liposomes for numerous years, aiming to enhance drug effectiveness and patient compliance, thereby offering improved treatment options for patients. Our development platform for injectable liposome formulations focuses on various indications including systemic fungal infections, tumors, vaccines, pain relief, etc. We are dedicated to advancing the field of injectable liposomes by harnessing state-of-the-art technologies, including degradable and injectable polymer liposome hydrogels, PEG-modified liposomes, and environmentally responsive liposomes. As a professional pharmaceutical R&D team with an exceptional platform and extensive experience, we consistently strive to offer patients more advanced and effective treatment options. By choosing to collaborate with us, you will benefit from our profound professional background, rich project experience, and efficient cooperation team while jointly advancing the field of liposome formulations.
Why Develop Injectable Liposome Formulations?
Liposome formulations present a novel approach to delivering drugs, offering numerous benefits such as the ability to carry high drug loads, excellent compatibility with biological systems, and controlled release of medications. These characteristics make liposome formulations highly promising for injectable applications in drug delivery and have been proven effective in delivering various types of drugs including proteins, peptides, and small molecules, making them applicable in treating a wide range of diseases like cancer, diabetes, and cardiovascular disorders. Additionally, liposome formulations can improve the availability and pharmacokinetic properties of drugs while enhancing their effectiveness and reducing adverse reactions.
The development process for injectable liposome formulations requires careful consideration of factors such as liposome composition, optimizing size parameters, and modifying surface properties that directly impact both efficacy and safety profiles. Therefore, achieving highly efficient injectable liposome formulations necessitates comprehensive studies on preparing liposomes and understanding drug delivery mechanisms while considering the physicochemical properties and biodistribution characteristics specific to each medication. Developing long-acting injectable liposome formulations holds immense significance in improving therapeutic outcomes while minimizing adverse reactions.
Explore Our Injectable Liposome Formulation Development Services
Our services cover the entire R&D process of injectable liposome development, including formulation design and screening, in vitro pharmacodynamic evaluation, testing phase and mass production challenges.
Formulation Design
In this service, we design the optimal according to the needs of customers through scientific technical means and conduct screening to ensure the quality and effect of the product.
In Vitro Pharmacodynamic Evaluation
In this stage, we employ advanced experimental techniques to assess the pharmacodynamic impact of the liposome, ensuring its efficacy in practical applications via vitro models such as cell models, animal models,etc.
Characterization Analysis
During the testing phase, we conduct comprehensive evaluations of the product, encompassing safety and efficacy assessments, in order to ensure compliance with national and industry standards.
Quality Standard Development
In the mass production stage, we guarantee product quality and stability through a scientific manufacturing process and rigorous quality control measures to meet customer requirements. We ensure smooth transfer of methods from the laboratory phase to the scale-up phase and control differences between batches.
Our Workflow of Injectable Liposome Formulation Development
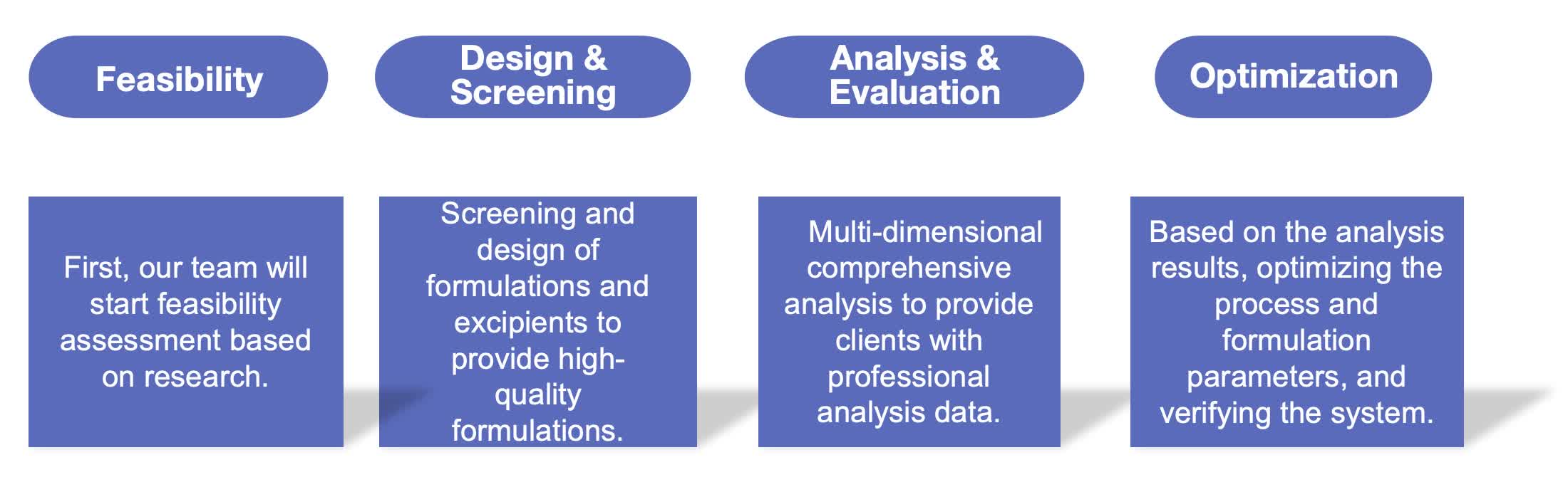 Fig.1 The workflow of injectable liposome formulation development. (CD Formulation)
Fig.1 The workflow of injectable liposome formulation development. (CD Formulation)
Our Platforms for Injectable Liposome Formulation Development
| Technology Platform |
Specific contents |
| Formulation Development Technology |
To design the optimal according to the needs of customers through scientific technical means and conduct screening to ensure the quality and effect of the product. |
| Lysolipid Thermally Sensitive Liposome (LTSL) Technology |
The primary application of LTSL is for the targeted release of drugs at elevated temperature sites. It is customary to employ lipids with phase transition temperatures ranging from 40°C to 45°C during the formulation process of these liposomes. |
| Non-PEGylated Liposome (NPL) Technology |
The NPL represent a distinctive drug delivery system that encompasses the advantages of PEGylated liposomes while mitigating the associated side effects of PEG. |
| Analysis Platform |
To provide an in-depth characterization and evaluation of the pharmacodynamics and stability of liposomes by meticulously analyzing their pharmacodynamic properties, stability, and potential applications in various therapeutic areas. |
Our Advantages in Injectable Liposome Formulation Development
- Comprehensive Expertise: Our team of highly skilled scientists and engineers has extensive experience in the development and manufacturing of injectable liposome formulations, ensuring that we can provide expert guidance and innovative solutions for even the most complex projects.
- Cutting-edge Technology: We continuously invest in the latest technology and equipment to ensure that our formulations are of the highest quality and exceed industry standards. Our facility is equipped with advanced liposome production systems, analytical instruments, and clean rooms, enabling us to deliver products that are both efficient and cost-effective.
- Customized Solutions: We understand that each client's needs are unique, which is why we offer tailored liposome formulations that are specifically designed to address the specific requirements of each project. Our team works closely with our clients to understand their objectives and develop formulations that deliver optimal results.
- Stringent Quality Control: We employ rigorous quality control measures throughout the development and manufacturing process. This ensures that our injectable liposome formulations are consistently safe, pure, and effective.
- Rapid Turnaround: Our efficient project management and streamlined processes enable us to provide rapid turnaround times for our clients, ensuring that their projects are completed on time and within budget.
Published Data
Technology: New Long-lasting Liposome Adenosine Drug Delivery Technology
Journal : Dsteoarthritis and Cartilage
IF : 7.0
Published : 2024
Results : In this study, the author used a novel longlasting formulations technology to deveop a injetable liposomal adenosine in the treatment of OA in a preclinical canine model. It was demonstrated that the liposome contributes to the upregulation of genes related to inflammation expression and contributes to the continuation of the inflammatory environment in osteoarthritis.
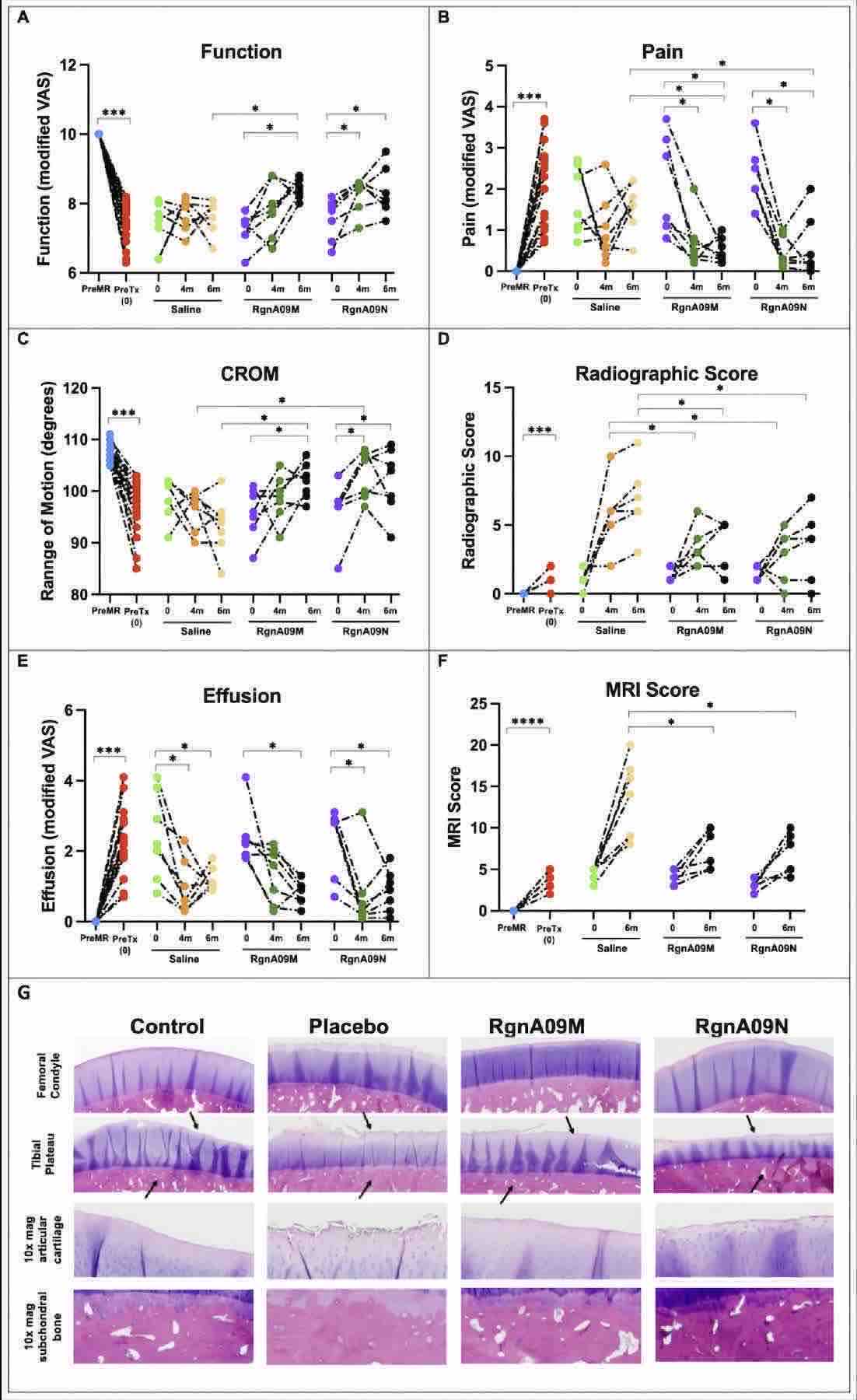 Fig.2 Pharmacodynamic evaluation. (Siddhesh R. Angle, et al., 2024)
Fig.2 Pharmacodynamic evaluation. (Siddhesh R. Angle, et al., 2024)
Our team at CD Formulation comprises a group of devoted researchers who are fully dedicated to addressing any obstacles that may emerge throughout the process of developing injectable liposomes. If you have any interest, please feel free to contact us without delay.
References
- Rahnfeld L, Luciani P. Injectable Lipid-Based Depot Formulations: Where Do We Stand? Pharmaceutics. 2020, 12(6): 567.
- Siddhesh R. Angle, Bruce N. Cronstein, et al. BozynskiIntra-articular Injections Of Liposomal Adenosine for Slowing Progression In A Preclinical Canine Model Of Osteoarthritis. Dsteoarthritis and Cartilage. 2024, 32(1): 13477.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services





