Custom Targeted Liposome Service
Inquiry
In recent years, the significance of molecular and diagnostic imaging in the management of various diseases, particularly in cancer treatment, has significantly escalated. In nanomedicine, substantial potential exists for integrating imaging and therapy. Engineered liposomes designed to selectively target tumor tissue can facilitate the delivery of drugs and imaging agents, thereby endowing diagnostic and therapeutic approaches with considerable promise in personalized medicine. CD Formulation is committed to advancing liposome targeting technology for in vivo imaging focusing on developing stable imaging liposomes.
Importance of Targeted Liposomes for In Vivo Imaging
Targeted liposomes refer to liposomes designed to deliver cargo or increase their retention in target cell populations through chemical interactions with cell surface molecules or other tissue-specific ligands. Targeted liposomes have a delivery advantage over non-targeted liposomes, not because they can better localize to the target site, but because once located at the target site, their interaction with the target cell population is increased. Currently, targeted liposomes are used for diagnosis, delivering magnetic resonance contrast agents and radioactive isotopes for magnetic resonance and nuclear medicine imaging. They have also been used for gene therapy to deliver various gene expression modifiers, including plasmids, antisense oligonucleotides, and short-interfering RNA.
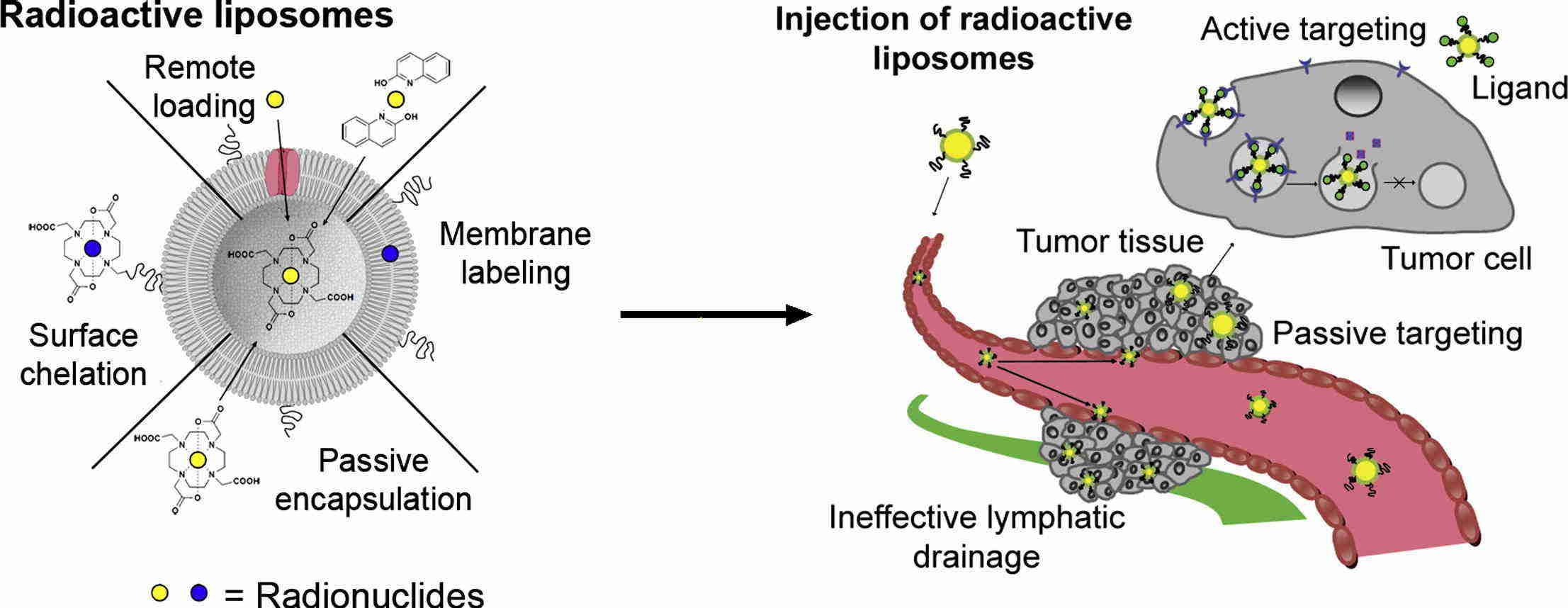 Fig.1 Mechanism of liposomal imaging of tumor tissue. (Petersen AL, et al., 2012)
Fig.1 Mechanism of liposomal imaging of tumor tissue. (Petersen AL, et al., 2012)
Explore Our Custom Targeted Liposome Service
Liposomes modification service
We provide specialized modification services, categorized into five main types based on the targeted modification reagent: peptide, antibody, carbohydrate, ligand, and nucleic acid aptamer.
Characterization service
The success of in vivo ultrasonic switchable fluorescence imaging heavily relies on the probe's physiological stability within biological tissues. We conduct in vitro and in vivo assessments including cell targeting, apoptosis and viability assays, phase transition, and ultrasound imaging tests of liposomes mediated by ultrasound, as well as in vivo phase transition and ultrasound imaging.
Our Capabilities for Targeted Liposome
| Techniques and Platforms |
Specifics |
| Targeting Techniques |
- Modification techniques include peptide targeting technique, antibody targeting technique, carbohydrate targeting technique, ligand targeting technique, and nucleic acid aptamer targeting technique.
|
| Characterization Platform |
- Various analytical instruments such as NucleoCounter NC-250 cell viability analyzer, Moxi GO II fast flow cytometer, ZERO fluorescent cell imaging System, etc.
|
Why Choose CD Formulation?
- Liposome Targeting Technique. By utilizing the potential of liposome targeting technology, we can precisely direct our focus to specific cells or tissues within the body. This maximizes the efficacy of in vivo imaging techniques and facilitates real-time monitoring of therapeutic effects.
- System Characterization Platform. Our platform offers researchers a wide range of analytical techniques to aid in stability and distribution characteristics of target liposomes at both cellular and in vivo levels.
- Professional Team. Our team comprises exceptional experts from fundamental medicine, molecular biology, chemistry, and pharmacy. It adopts a multidisciplinary approach that integrates artificial intelligence and materials chemistry to deliver innovative solutions.
Published Data
Technology: Endoglin-based in vivo near-infrared fluorescence imaging of tumor models in mice using an activatable liposome technique
Journal: Biochimica et Biophysica Acta (BBA) - General Subjects
IF: 2.8
Published: 2018
Results: In this study, the authors investigated the role of encapsulated DY-676-COOH in liposomes binding to single-chain antibody fragments after optimization. Due to the low homology of amino acid sequences between human and mouse endothelial glycoproteins, cross-reactivity is negligible, distinguishing the targets of the two antibody fragments. Validation of tumor and vascular endothelial factor targeting using mEnd-IL and hEnd-IL demonstrated clear detection of corresponding cells in both in vivo and ex vivo settings, as well as xenograft tumor models of human breast and fibrosarcoma. However, hEnd-IL exhibited a high fluorescence intensity, leading to a higher contrast ratio with the background in macroscopic optical imaging. In contrast, mEnd-IL accumulated in organ vasculature and showed a lower signal. Histological examination using co-focused microscopy revealed that fluorescence from mEnd-IL was located within the tumor and aforementioned-organ vasculature, while hEnd-IL mainly accumulated within tumor cells.
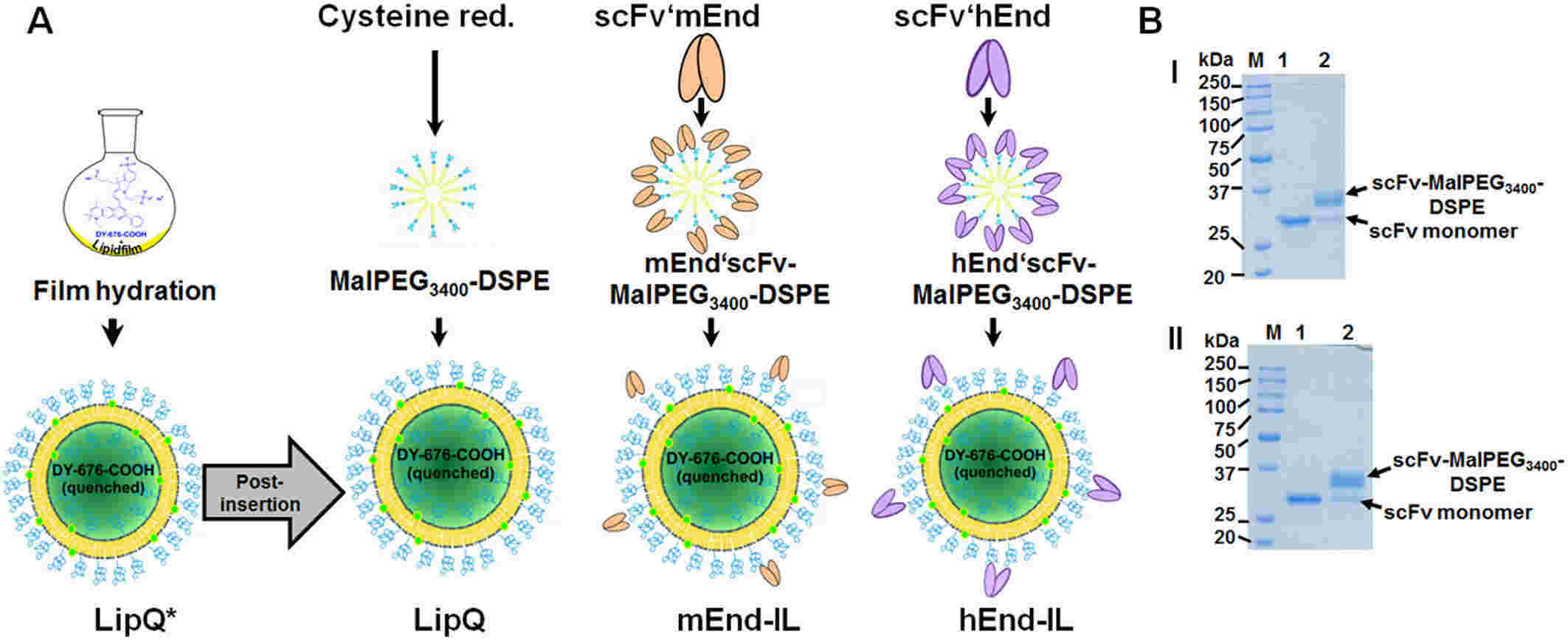 Fig.2 Preparation scheme for endoglin-targeting activatable liposomes. (Tansi FL, et al., 2018)
Fig.2 Preparation scheme for endoglin-targeting activatable liposomes. (Tansi FL, et al., 2018)
As a leading provider in the nanotechnology field, CD Formulation is committed to developing imaging liposomes for in vivo imaging applications, tailored to meet the R&D needs of pharmaceutical companies and institutions. Feel free to contact us if you need any assistance.
References
- Petersen AL, Hansen AE, et al. Liposome imaging agents in personalized medicine. Advanced drug delivery reviews. 2012 Oct 1; 64(13): 1417-35.
- Tansi FL, Rüger R, et al. Endoglin based in vivo near-infrared fluorescence imaging of tumor models in mice using activatable liposomes. Biochimica et Biophysica Acta (BBA)-General Subjects. 2018 Jun 1; 1862(6): 1389-400.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Mechanism of liposomal imaging of tumor tissue. (Petersen AL, et al., 2012)
Fig.1 Mechanism of liposomal imaging of tumor tissue. (Petersen AL, et al., 2012) Fig.2 Preparation scheme for endoglin-targeting activatable liposomes. (Tansi FL, et al., 2018)
Fig.2 Preparation scheme for endoglin-targeting activatable liposomes. (Tansi FL, et al., 2018)
