Custom Radiolabeled Liposome Service
Inquiry
Radiolabeled liposomes have gained significant attention as versatile carriers for the targeted delivery of therapeutic and diagnostic agents, enhancing precision in treatment and imaging. CD Formulation specializes in leveraging advanced technologies and expertise to support the development of high-performance radiolabeled liposomes tailored for various applications.
Why are Radiolabeled Liposomes Needed?
Biomedical imaging techniques play a crucial role in enhancing cancer treatment efficacy and elevating patient survival rates. Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) stand out as the preferred modalities owing to their exceptional sensitivity for deep tissue visualization. In nuclear imaging applications, timely administration of radioactive tracers is essential for precise localization and monitoring of tumors or anomalies during both clinical trials and routine practice. These radioactive tracers—also referred to as nuclear imaging probes—comprise varying radioactive isotopes based on decay pathways, timing characteristics, emitted energy levels, and depth of tissue penetration. The resultant images from these advanced technologies enable comprehensive assessment of physiological processes alongside metabolic substances within specific regions while facilitating quantitative or semi-quantitative evaluations thereof. Furthermore, in the realm of nuclear medicine, radiopharmaceuticals represent amalgamations of biologically pertinent molecules combined with radioactive isotopes utilized for visualizing organ/tissue distributions and detecting metabolic activities via PET or SPECT methodologies.
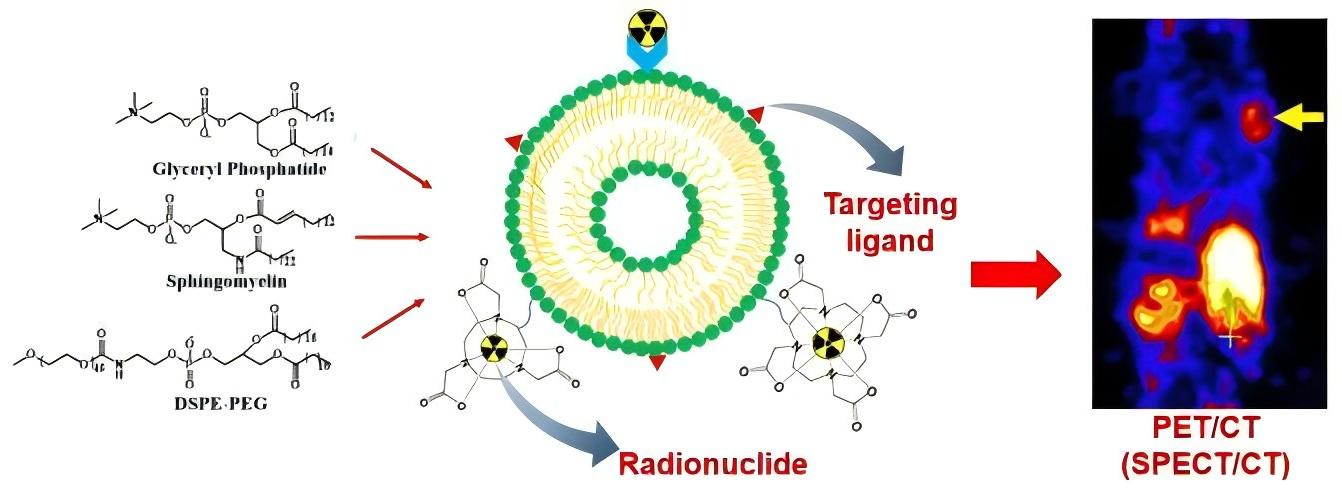 Fig.1 Typical liposome modifications for nuclear imaging. (Low HY, et al., 2023)
Fig.1 Typical liposome modifications for nuclear imaging. (Low HY, et al., 2023)
Our Radiolabeled Liposome Customization Service
Radiolabeled Liposome formulation development service
During this service, we evaluate appropriate radionuclides and radiolabeling techniques based on the intended application and research objectives of the radionuclides, with particular attention to ensuring that the radionuclide's half-life is compatible with the biological half-life of the liposomes.
Customization of radiolabeled liposomes for different imaging modes
The sensitivity and penetration of various imaging systems differ; for example, SPECT and PET are commonly used to visualize metabolic and physiological processes but offer lower resolution in cell anatomical structures. Conversely, CT or MRI compensate for these limitations. Given the high selectivity of radioactive elements for different imaging techniques, we provide liposome products with radioactive labeling suitable for various imaging systems through this service.
In vivo validation service
Before in vivo validation, we need to conduct a comprehensive assessment, including drug loading and the half-life compatibility of liposomes with radioactive elements. During the in vivo validation experiment, our primary focus is on monitoring blood clearance rate, in vivo drug release kinetics, bioavailability, and targeted drug delivery.
Our Platforms for Radiolabeled Liposome Customization
| Techniques and Platforms |
Specifics |
| Radiolabeled Liposome Techniques |
- Isotope labelling (including 18F, 64Cu, 124I, 68Ga, 89Zr, 99mTc, 111In, 67Ga, 123I, 177Lu, etc.).
- Radiolabeling methods include passive encapsulation, membrane labeling, remote labeling-ionophore, remote labeling-lipophilic chelator, and remote labeling-surface chelator.
|
| Characterization analysis platform |
- Physical and chemical analysis including drug loading and half-life compatibility of liposome with radioactive elements.
- In vivo experiments such as blood clearance rate, in vivo drug release kinetics, bioavailability, targeted drug delivery, etc.
|
Why Choose CD Formulation?
- Liposome radioactive element labeling techniques. Our liposome radioactive element labeling techniques involve the precise labeling of liposomes with radioactive elements and selecting suitable radioactive elements, allowing for targeted delivery and imaging in medical applications.
- An advanced feature analysis platform for radiolabeled liposomes. This platform offers state-of-the-art imaging and analytical techniques to investigate the pharmacokinetics, biodistribution, and cellular uptake of radiolabeled liposomes in preclinical and clinical settings. Researchers can utilize this platform to gain insights into the efficacy and safety of liposomal formulations, assess their potential for targeted drug delivery, and optimize their design for specific therapeutic applications.
- The proficient teams. The development of radioactive liposomes is led by a highly skilled team of experts specializing in molecular biology, chemistry, and pharmacy. In close collaboration with regulatory specialists, they navigate the intricate landscape of clinical translation to ensure strict compliance with safety standards.
Published Data
Technology: Long-circulating liposomes labeled technique with 111In-oxine
Journal: Journal of Controlled Release
IF: 10.8
Published: 2015
Results: Long-circulating liposomes (LCLs) are frequently utilized as carriers for water-soluble drugs to enhance their therapeutic effectiveness. To monitor the behavior of these LCLs in living organisms, they can be tagged with 111In-oxine. The conventional method involves encapsulating DTPA in the aqueous phase of the LCL (DTPA-LCL) for labeling. Alternatively, DTPA-bound DSPE can be integrated into the lipid bilayer and labeled with 111InCl3 to form DTPA-DSPE LCL (DTPA-DSPE LCL). This study compares the in vivo performance of DTPA-DSPE LCL with that of DTPA-LCL in mice. Labeling DTPA-DSPE LCL with 111InCl3 is a robust, straightforward, and rapid procedure.
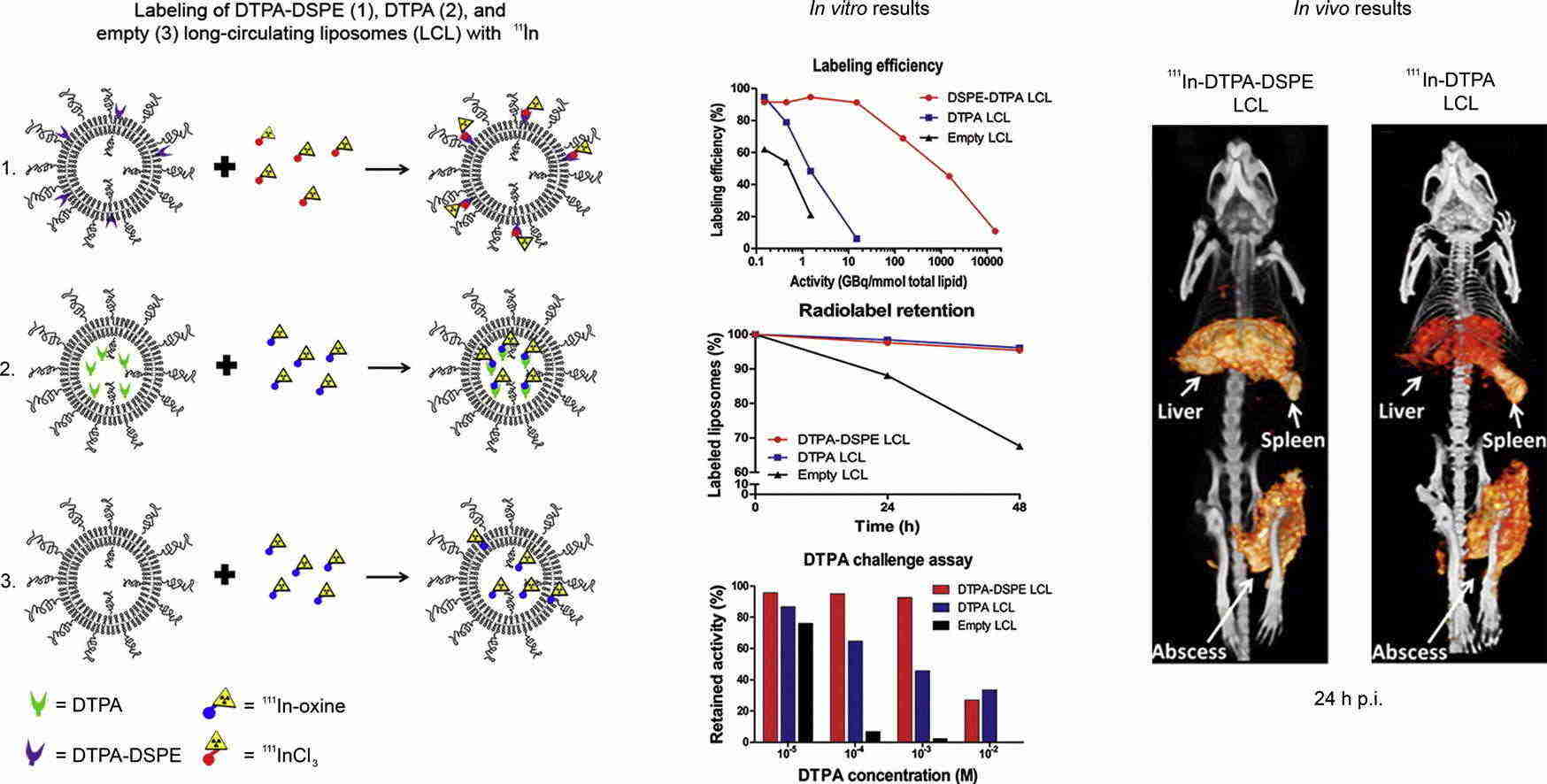 Fig.2 Images of near-infrared in vivo imaging system. (Van Der Geest T, et al., 2015)
Fig.2 Images of near-infrared in vivo imaging system. (Van Der Geest T, et al., 2015)
As a leader in nanoparticles, CD Formulation is dedicated to delivering exceptional radiolabeled liposomes for in vivo imaging applications. Please do not hesitate to contact us if you require any assistance.
References
-
Low HY, Yang CT, et al. Radiolabeled Liposomes for Nuclear Imaging Probes. Molecules. 2023 Apr 28; 28(9): 3798.
- Van Der Geest T, Laverman P, et al. Comparison of three remote radiolabelling methods for long-circulating liposomes. Journal of Controlled Release. 2015 Dec 28; 220: 239-44.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 Typical liposome modifications for nuclear imaging. (Low HY, et al., 2023)
Fig.1 Typical liposome modifications for nuclear imaging. (Low HY, et al., 2023) Fig.2 Images of near-infrared in vivo imaging system. (Van Der Geest T, et al., 2015)
Fig.2 Images of near-infrared in vivo imaging system. (Van Der Geest T, et al., 2015)
