Proliposomes, defined as carb carriers wrapped in phospholipids, are formed by adding water. With our expertise and technology accumulation in the field of liposomes, the scientific research team at CD Formulation can provide professional solutions for customizing and characterizing proliposomes.
What Are Proliposomes?
Liposomes represent the most promising and widely utilized drug delivery systems. Nevertheless, liposomes are plagued by issues of poor stability, leading to potential storage challenges. To address these stability concerns, precursor liposomes are characterized as dry, free-flowing particles with a dispersion system that can promptly form a liposomal suspension upon contact with water. Precursor liposomes contribute to enhanced bioavailability.
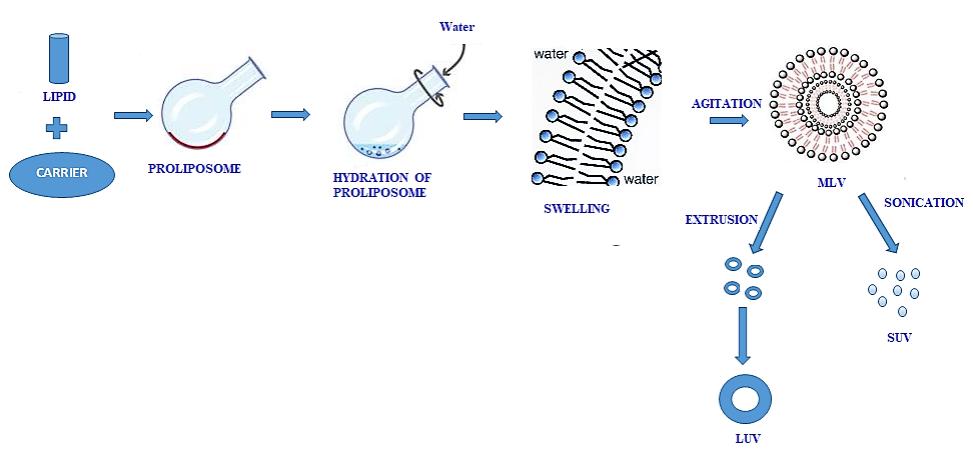 Fig.1 The mechanism of liposome formation from Proliposomes. (Xue-Qin Wei, et al., 2020)
Fig.1 The mechanism of liposome formation from Proliposomes. (Xue-Qin Wei, et al., 2020)
Explore Our Custom Proliposome Service
Screening Methods for Proliposome Customization
There are various methods for preparing proliposomes. Due to diverse factors such as vesicle size, size distribution, encapsulation capacity, and retention of contents, it is necessary to select the appropriate method. We screen drugs based on their physicochemical properties, type of phospholipid required, target particle size range, and preparation method.
Proliposome Characterization
Primarily for the characterization of services including, but not limited to, particle size, zeta potential, encapsulation efficiency, drug loading, etc.
Hydration Study and Vesicle Formation
It is important to determine the formation of liposomal vesicles following hydration of the proliposomal formulation in vitro. We confirm the formation of vesicles with light microscopy: The liposome suspension should be applied onto a glass slide and air-dried at room temperature. Subsequently, the resulting dry film of the liposome suspension should be examined for vesicle formation.
In Vitro Drug Release from Proliposomes
We conducted in vitro drug release studies of proliposomes using various techniques, such as USP dissolution apparatus Type I, Franz diffusion cells, dialysis tubes, reverse dialysis, glass paper diffusion membranes, transparent diffusion cells, and spectral molecular porous membrane tubes. In vitro skin permeation studies are conducted on the back skin of albino rabbits, albino rabbits, female Spragge-Dawley rats (7-9 weeks old), and Wistar rats (7-9 weeks old).
Our Capabilities for Customizing Proliposomes
| Items |
Detailed Information |
| Physicochemical characterization |
- Including particle size, potential, encapsulation rate, drug loading, morphology, etc.
|
| Hydration study and vesicle formation |
- To determine the formation of liposomal vesicles following hydration of the proliposomal formulation.
- To confirm the formation with light microscopy.
|
| In vitro drug release study |
- Various techniques, such as USP dissolution apparatus Type I, Franz diffusion cells, dialysis tubes, reverse dialysis, glass paper diffusion membranes, transparent diffusion cells, and spectral molecular porous membrane tubes.
|
| In vitro skin permeation study |
- In vitro skin permeation studies are conducted on animal skins.
- We can offer back skin of albino rabbits, albino rabbits, female Spragge-Dawley rats (7-9 weeks old), Wistar rats (7-9 weeks old), etc.
|
Our Key Advantages in Customizing Proliposomes
- Advanced platforms. Our platform supports the development and customization of multiple proliposomes, based on a various preparation processes and methods.
- Comprehensive analysis service. From physical and chemical properties, in vitro release studies, to in vivo evaluation, we guarantee to provide our customers with highly stable, effective, and less toxic side effects of the proliposomes.
- Skilled teams. Our R&D teams consist of many scientists with multi-disciplinary backgrounds who are well-versed in the development and evaluation of multiple proliposomes. At present, we have successfully helped many customers customize their proliposomes.
Published Data
Technology: Proliposome powder or tablets for generating inhalable liposomes using a medical nebulizer technique
Journal: Journal of Pharmaceutical Investigation
IF: 5.5
Published: 2021
Results: In this study, the original liposome powder was prepared by slurry method with sorbitol or mannitol carbohydrate as carrier Lipid phase. The original liposome powder was compressed into tablets, and the liposome was prepared from the original liposome powder, or the tablets were atomized in the nebulizer storage for subsequent atomization. This study shows that proliposome tablets can disintegrate in Pari-LC Sprint atomizers produce inhalable aerosols with high drug yields and can therefore be mass-produced to overcome storage problems related to powder formulation.
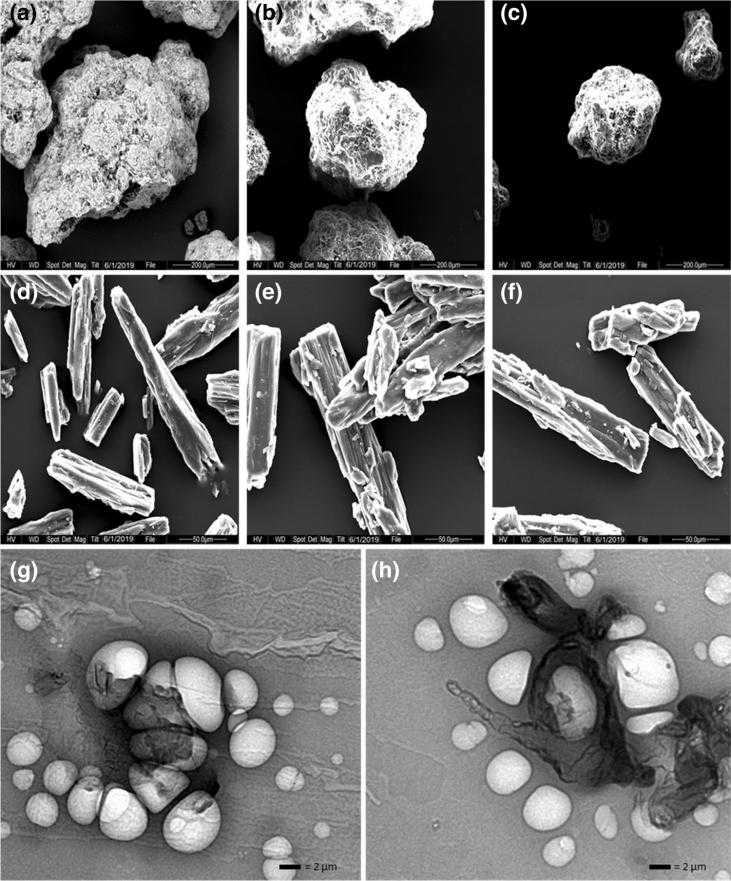 Fig.2 SEM images of coarse carbohydrate carrier and proliposome powder. (Khan, I, et al., 2021)
Fig.2 SEM images of coarse carbohydrate carrier and proliposome powder. (Khan, I, et al., 2021)
CD Formulation has extensive project experience and expertise in the customization of proliposomes. If you need any help, please do not hesitate to contact us.
References
- Khan, I., Yousaf, S., et al. Proliposome powder or tablets for generating inhalable liposomes using a medical nebulizer. J. Pharm. Investig. 2021, 51, 61–73.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 The mechanism of liposome formation from Proliposomes. (Xue-Qin Wei, et al., 2020)
Fig.1 The mechanism of liposome formation from Proliposomes. (Xue-Qin Wei, et al., 2020) Fig.2 SEM images of coarse carbohydrate carrier and proliposome powder. (Khan, I, et al., 2021)
Fig.2 SEM images of coarse carbohydrate carrier and proliposome powder. (Khan, I, et al., 2021)
