Custom Multivesicular Liposome Service
Inquiry
Over the past decade, multivesicular liposomes (MVL) have shown significant progress in continuous drug delivery, offering high encapsulation efficiency, structural stability, size variability, and diverse delivery routes. CD Formulation leverages cutting-edge technologies and expertise to develop high-performance, intelligent multivesicular liposomes to meet specific research needs.
Mechanism of Multivesicular Liposomes
Multivesicular liposomes (MVL) are micrometer-sized spherical particles composed of non-centrosymmetric lipid bilayers, enclosing drug molecules within their internal aqueous compartments. This polyhedral structure ensures the integrity and sustained release of small molecules, peptides, or proteins during non-vascular administration. The release of multivesicular liposomes follows a three-phase pattern. Initially, there is the rapid release of the free drug followed by a sudden burst of the drug. This is then succeeded by a slower lag phase in the release rate, attributed to the reorganization of the lipid membrane. At temperatures above the lipid phase transition, membrane fusion occurs, leading to the collapse of the honeycomb structure. The final stage involves secondary release caused by erosion due to lipid hydrolysis.
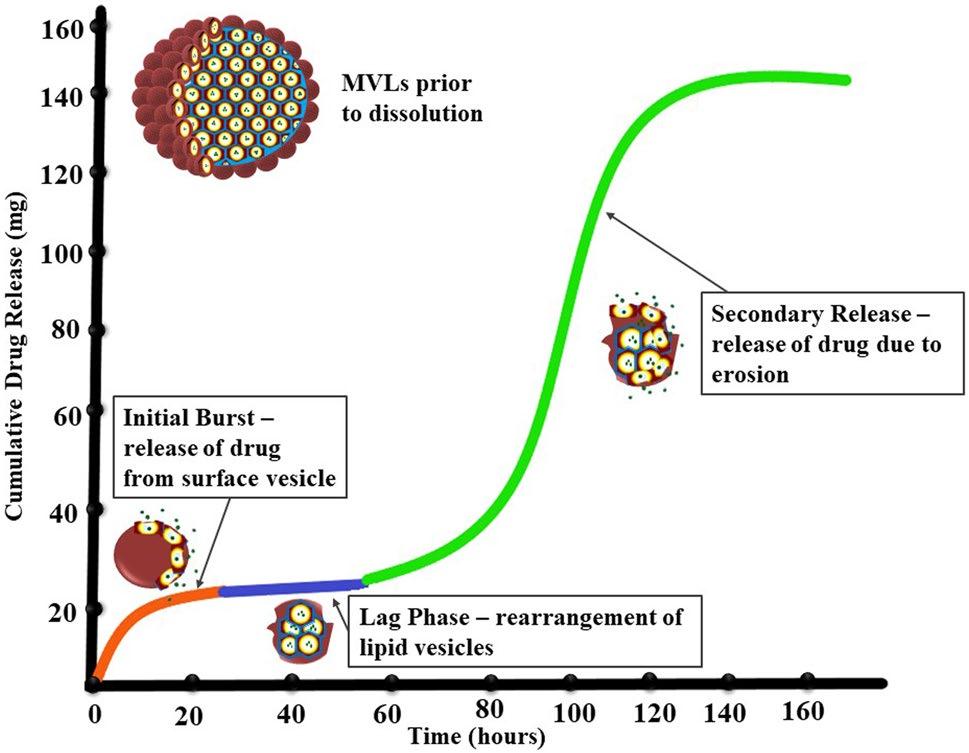 Fig.1 Schematic plot of the speculated drug release mechanism of MVL particles. (Chaurasiya, A., et al., 2022)
Fig.1 Schematic plot of the speculated drug release mechanism of MVL particles. (Chaurasiya, A., et al., 2022)
Our Multivesicular Liposome Customization Service
Formulation study and parameter optimization
We offer formulation studies and parameter optimization such as charged phospholipids, cholesterol, neutral lipids, process temperature, process time and shear, etc.
Study on the route of administration
Multivesicular liposome drug delivery can be achieved via various administration routes, such as intramuscular, subcutaneous, intravenous, subarachnoid, intra-articular, intravitreal, and epidural injections. We can provide research on diverse methods of drug delivery.
Characterization analysis
We provide physical and chemical characterization (particle size, charge distribution, charge load and encapsulation rate, viscosity, rheology, appearance, internal and external pH, internal and external ionic strength, stability, etc.). In vitro characterization services include cell uptake rate assay, cytotoxicity assay, and evaluation of tumor cell-targeting ability. In vivo characterization services such as in vivo distribution analysis, in vivo bioactivity analysis, in vivo stability analysis, etc.
Our Platforms for Multivesicular Liposome Customization
| Techniques and Platforms |
Specifics |
| Double emulsification technique |
- The technique from laboratory to commercial manufacturing.
|
| Spray atomization technique |
- The primary benefit of this technology lies in its improved integration of all stages and the elimination of organic solvents.
|
| Characterization techniques |
- Lipid characterization.
- Vesicle size and size distribution (including Laser diffraction, confocal microscopy, and cryo-SEM).
- Morphology (including microscopy and cryo-SEM).
- Encapsulation efficiency and free drug.
- Residual solvent content.
- Internal and external pH.
- Internal and external ionic strength.
|
| In vitro and In vivo characterization platform |
- In vitro characterization includes cell uptake rate assay, cytotoxicity assay, and evaluation of tumor cell-targeting ability.
- In vivo characterization such as distribution analysis, bioactivity analysis, stability analysis, etc.
|
Why Choose CD Formulation?
- Top multivesicular delivery platform. The platform supports the development of innovative multi-capsule liposome products, allows the study of their reaction mechanisms, and provides customers with system customization services including formulation development, mechanism research, in vitro and in vivo release studies, etc.
- Techniques for multivesicular liposomes. We possess sophisticated technology for multi-vesicular liposomes, encompassing preparation and characterization techniques, to ensure the delivery of high-quality customized products to our clients.
- The proficient teams. The technical team comprises seasoned and highly skilled experts proficient in multivesicular liposome technology, with diverse multidisciplinary backgrounds and extensive knowledge in the field.
Published Data
Technology: Multivesicular liposomes for the sustained release of angiotensin I-converting enzyme (ACE) inhibitory peptides techniques
Journal: Molecules
IF: 4.2
Published: 2024
Results: In this paper, the authors aim to add water-soluble substances to MVL to enhance slow release and prevent drug destruction and elucidate the functions and mechanisms of different components. In vitro controlled release studies show that MVLs have good controlled release performance and thermal stability. Angiotensin-converting enzyme (ACE) inhibitory peptide (AP) -MVLs reduced angiotensin-converting enzyme (ACE) inhibitory activity by only 2.84% after oral administration and 5.03% after passing through the stomach, which could be used as a promising sustained-release drug delivery system.
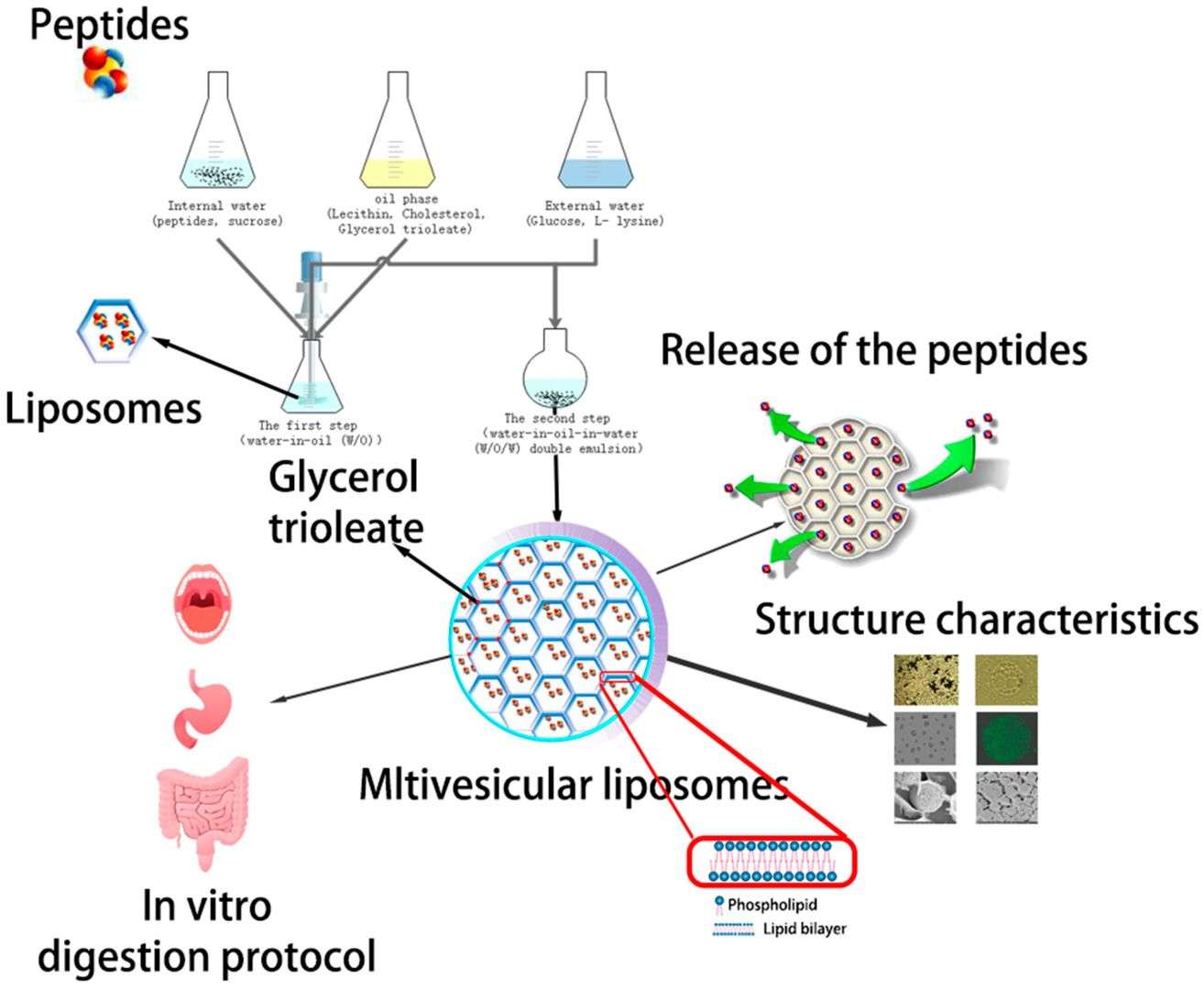 Fig.2 Schematic representation of the Angiotensin-converting enzyme (ACE) inhibitory peptide (AP) -MVLs. (Li N, et al., 2019)
Fig.2 Schematic representation of the Angiotensin-converting enzyme (ACE) inhibitory peptide (AP) -MVLs. (Li N, et al., 2019)
As a leading company in nanoparticle development, CD Formulation is dedicated to providing excellent multivesicular liposome products. Please do not hesitate to contact us if you require any assistance.
References
-
Chaurasiya, A., Gorajiya, A., et al. A review on multivesicular liposomes for pharmaceutical applications: preparation, characterization, and translational challenges. Drug Deliv. and Transl. 2022. Res. 12, 1569–1587
- Li N, Shi A, et al. Multivesicular Liposomes for the Sustained Release of Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides from Peanuts: Design, Characterization, and In Vitro Evaluation. Molecules. 2019; 24(9): 1746.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 Schematic plot of the speculated drug release mechanism of MVL particles. (Chaurasiya, A., et al., 2022)
Fig.1 Schematic plot of the speculated drug release mechanism of MVL particles. (Chaurasiya, A., et al., 2022) Fig.2 Schematic representation of the Angiotensin-converting enzyme (ACE) inhibitory peptide (AP) -MVLs. (Li N, et al., 2019)
Fig.2 Schematic representation of the Angiotensin-converting enzyme (ACE) inhibitory peptide (AP) -MVLs. (Li N, et al., 2019)
