Custom Long-circulating Liposome Service
Inquiry
Long-circulating liposomes refer to liposomes that prevent plasma proteins from adsorbing on their surface in vivo, thus avoiding rapid phagocytosis by the mononuclear macrophage system and prolonging blood circulation time. This is conducive to increasing the relative accumulation of liposomes at pathological sites due to the prevention of modulatory and physicochemical actions. With our expertise and technology accumulation in the field of liposomes, the scientific research team at CD Formulation can provide professional solutions for customizing and characterizing long-circulating liposomes.
What Are Long-circulating Liposomes?
Liposomes have been extensively researched as vehicles for delivering drugs, and numerous previous studies have shown their effectiveness in encapsulating drugs and minimizing drug side effects. In many instances, liposomal drugs are transported through the bloodstream. The stability, clearance, and distribution of liposomes in the blood rely on their composition, size, and charge. Conventional liposomes are quickly removed from the bloodstream by being absorbed by mononuclear phagocytic system (MPS) cells. Long-circulating liposomes can address this issue by attaching polyethylene glycol (PEG) to liposomal phospholipids to extend their lifespan and shield them from regulation and capture by the reticuloendothelial system (RES). Covalently bonding liposomes to PEG shields the active part of the recipient's immune system, thereby reducing immunogenicity and antigenicity. It also alters the physical and chemical properties of the active part, including changes in hydrodynamic size, which further reduces renal clearance and prolongs circulation time. Additionally, it converts hydrophobic drugs into hydrophilic ones, decreasing the need for frequent administration. Moreover, due to the leaky nature of tumor blood vessels, nanoparticles with prolonged circulation times demonstrate enhanced penetration and retention (EPR) as they slowly accumulate in tumor tissues.
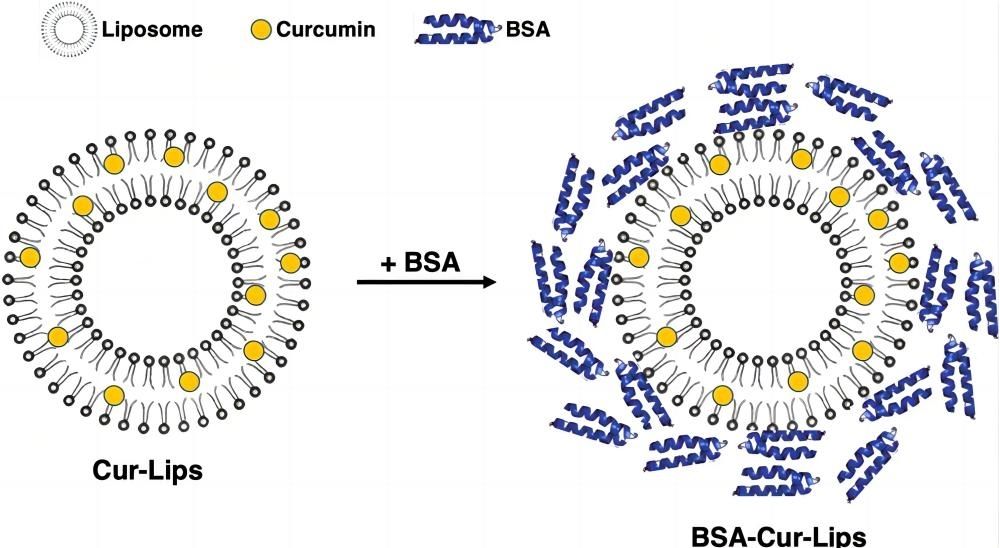 Fig.1 Curcumin liposome long cycle drug delivery system. (Xue-Qin Wei, et al., 2020)
Fig.1 Curcumin liposome long cycle drug delivery system. (Xue-Qin Wei, et al., 2020)
Explore Our Custom Long-circulating Liposome Service
Customization of Long-circulating with Different Chain Lengths
The most extensively studied long-circulating liposome systems are PEG-liposomes. This liposome type is created by grafting low-molecular poly(ethylene glycol) (PEG) to the lipid bilayer surface. In addition, we can provide modification services for derivatives, and colleagues can provide PEG-modified liposomes with different chain lengths.
Accelerated Blood Clearance (ABC) Phenomenon Studies
PEG-liposomes may lose their long-circulation ability when injected repeatedly, a phenomenon known as "accelerated blood clearance (ABC)", a phenomenon that limits the effectiveness and safety of pegylated liposomes and seriously affects their clinical use. With this service, we help our customers explore more alternative long-circulating liposomes to PEG modifications that avoid the ABC effects caused by such PEG liposomes. Alternatives are currently available, such as saponin derivatives, Polycarboxybetaine (PCB), branched-chain PEG, poly sarcosine (PSar), poly glycerin, polyhydroxyethyl-1-asparagine (PHEA), poly ethylpyrrolidone (PVP), poly (N), N-dimethyl acrylamide) (PDMA), poly (N-acrylomorpholine) (PAcM), poly [N- (2-hydroxypropyl)) methylacrylamide] (HPMA) and poly (2-methyl-2-oxazoline) (PMOX), etc.
Long-circulating Liposome Characterization
For long-circulating liposomes, stability, and particle size are the most important characteristics of physical and chemical properties, which are about the efficacy of the long cycle.
- Formulation stability study
To prevent drug leakage from liposomes both upon storage and after injection in the circulation, the stability of the drug-containing liposome formulation must be investigated and optimized.
Liposomes must be sufficiently small and possess a prolonged circulation half-life to facilitate extensive penetration beyond the pathological site.
Our Capabilities for Customizing Long-circulating Liposomes
| Items |
Detailed Information |
| Physicochemical characterization |
- Including particle size, potential, encapsulation rate, drug loading, stability, etc.
|
| Accelerated blood clearance (ABC) phenomenon studies |
- To explore more alternative long-circulating liposomes to PEG modifications that avoid the ABC effects caused by such PEG liposomes. Alternatives such as saponin derivatives.
|
| Screening of different chain lengths |
- Efficacy screening for different chain lengths.
- To help customers choose an effective chain length, too long or too short is not appropriate.
|
Our Key Advantages in Customizing Long-circulating Liposomes
- Advanced platforms. We have established a customized platform for the development of long-cycle liposomes, which supports the screening of PEG and derivatives of different chain lengths as accessories.
- Comprehensive analysis service. We offer a specialized platform for the characterization of long-circulating liposomes, encompassing analysis of their physical and chemical properties as well as in vivo and in vitro bioactivity assessments, to ensure the delivery of highly tailored long-circulating liposome products to our customers.
- Skilled teams. Our research and development team consists of numerous scientists with diverse backgrounds in various disciplines, who possess extensive expertise in the development, mechanisms of action, and thorough evaluation of long-circulating liposomes. They are proficient in addressing customized inquiries from customers regarding long-acting liposomes.
Published Data
Technology: Long-circulating thermosensitive liposomes technique
Journal: Int J Nanomedicine
IF: 8.0
Published: 2020
Results: In this study, the LCTL containing L-OHP (L-OHP/LCTL) was prepared by the reverse-phase evaporation (REV) method, and its physical properties, including encapsulation rate (EE), particle size, potential, and stability, were evaluated. The release behavior, cytotoxicity, and in vivo were also evaluated. In vivo results showed that LCTL could target the tumor and enhance the retention time for more than 24 hours, thus enhancing the antitumor effect on 4T1 tumor-bearing mice. These results suggest that LCTL not only has the effect of prolonging circulation time but also can enhance accumulation and achieve selective release at the tumor site. In conclusion, LCTL can be used as a promising vector for oxaliplatin therapy in solid tumors.
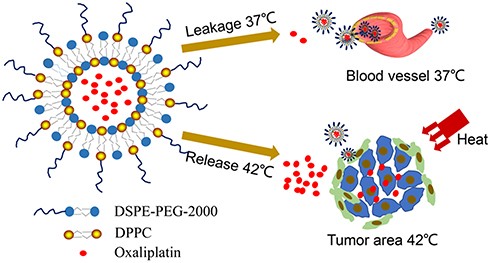 Fig.2 Scheme illustration of long cycle heat-sensitive liposomes for oxaliplatin targeted drug delivery. (Li Y, et al., 2020)
Fig.2 Scheme illustration of long cycle heat-sensitive liposomes for oxaliplatin targeted drug delivery. (Li Y, et al., 2020)
CD Formulation has extensive experience in the development and customization of long-circulating liposomes. If you need any help, please do not hesitate to contact us.
References
- Xue-Qin Wei, Kai Ba. Construction a Long-Circulating Delivery System of Liposomal Curcumin by Coating Albumin. ACS Omega, 2020, 5 (27), 16502-16509.
- Li Y, Xu P, et al. Long-Circulating Thermosensitive Liposomes for the Targeted Drug Delivery of Oxaliplatin. Int J Nanomedicine. 2020; 15: 6721-6734
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Curcumin liposome long cycle drug delivery system. (Xue-Qin Wei, et al., 2020)
Fig.1 Curcumin liposome long cycle drug delivery system. (Xue-Qin Wei, et al., 2020) Fig.2 Scheme illustration of long cycle heat-sensitive liposomes for oxaliplatin targeted drug delivery. (Li Y, et al., 2020)
Fig.2 Scheme illustration of long cycle heat-sensitive liposomes for oxaliplatin targeted drug delivery. (Li Y, et al., 2020)
