Custom Immunoliposome Service
Inquiry
Liposomes are a new artificial method of producing spherical vesicles, which consist of a bilayer of lipid molecules, and the surface-exposed antibody markers provide a variety of diagnostic advantages. Liposomes lack the ability to target drug delivery to specific cell types, while immunoliposomes can achieve targeted delivery by binding some antibody fragments to their surface. Immunoliposomes are considered to be attractive drug targeting vectors for cancer therapy. With our expertise and technology accumulation in the field of liposomes, the scientific research team at CD Formulation can provide professional solutions for customizing and characterizing diverse immunoliposomes.
What Are Immunoliposomes?
Immunoliposomes are liposomes that have been functionalized by attaching monoclonal antibodies or their fragments (Fabs) and scFvs to the liposomes through covalent binding of the antibody, or its fragment, to the carrier lipid or by chemically modifying the antibody to increase its hydrophobicity, resulting in a higher affinity for the liposome carrier bilayer. After binding to tumor cells, drugs encapsulated in immunoliposomes can be delivered through four different mechanisms: (1) adsorbed on the cell surface; (2) Fusion with cell membrane; (3) Exchange lipid components with cell membranes; and (4) endocytosis pathway. For receptor-mediated endocytosis, drug-containing liposome must be able to penetrate the cell interior and bypass lysosomal degradation in order to effectively target intracellular compartments.
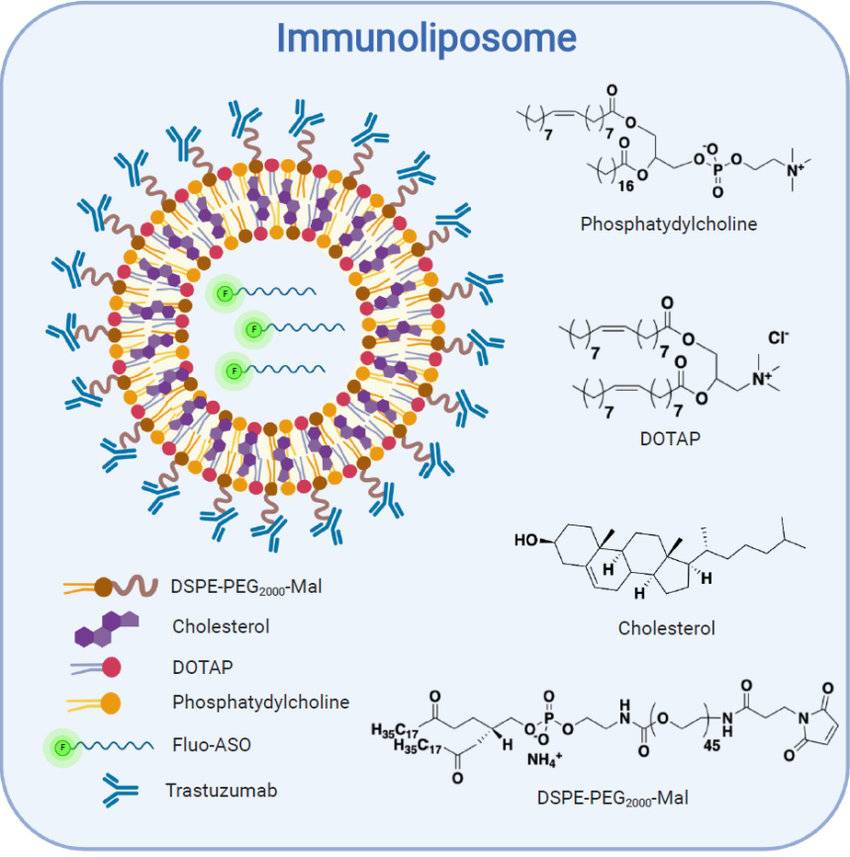 Fig.1 Schematic representation of the immunoliposome structure and composition. (Sicard, Guillaume., et al., 2020)
Fig.1 Schematic representation of the immunoliposome structure and composition. (Sicard, Guillaume., et al., 2020)
Explore Our Custom Immunoliposome Service
Screening of Antibodies/Targeting ligands
The main protein types used as targeting ligands are antibodies and antibody fragments (Fab or scFv). Immunoglobulins (IgG1, IgG2a, IgG2b) are the most common ones used in targeted drug delivery. There are five main types of immunoglobulins, including IgA, IgD, IgE, IgG, and IgM. IgG is the main type of human immunoglobulin and can be further divided into four subtypes: IgG1, IgG2, IgG3, and IgG4. This service is designed to help customers select suitable ligands.
Liposomes Conjugated to Antibody Fragments
The advantages of using smaller functional antigen binding fragments of antibodies include avoiding the possibility of initiating an immune response, higher surface load due to reduced crowding with minimal perturbation of the shape, size, and function of the nanoparticle and antibody fragment itself, and efficient delivery of the drug to the target site. We can provide modifications of antibody fragments on amino, carboxyl, and thiol groups, with lysine, cysteine, and glutamate/aspartic acid residues being the most common modification sites.
Liposomes Conjugated to Monoclonal Antibodies
The conjugated methods we typically provide are based on three primary reactions: the condensation of activated carboxyl and amine groups to form amide bonds, the reaction between pyrimidinedithiol and thiols to form disulfide bonds, and the reaction between maleimide derivatives and thiols to form thioether bonds.
Stimuli-responsive Immunoliposomes
A novel approach has been developed for the construction of intelligent multifunctional immunoliposomes, which are capable of releasing encapsulated drugs upon stimulation. We provide tailored immunoliposomes, including pH-responsive, enzyme-responsive, redox-responsive, and magnetic-responsive types.
Our Capabilities for Customizing Immunoliposomes
| Items |
Detailed Information |
| Lipid Nanoparticle Coupling Technology |
- Liposomes conjugated to antibody fragments technique
- Liposomes conjugated to monoclonal antibodies: conjugation by thiol group, conjugation by amino acid and carboxylic acid residue.
|
| Intelligent Multifunctional Immunoliposomes |
- Stimuli-responsive immunoliposomes types:
pH-responsive, enzyme-responsive, redox-responsive, and magnetic-responsive types. |
| Immunoliposome Characterization |
- Physicochemical properties: particle size, potential distribution, encapsulation rate, and drug loading.
- In vitro and in vivo characterization: in vitro release, in vivo distribution, in vivo targeting, half-life, etc.
|
Our Key Advantages in Customizing Immunoliposomes
- Advanced conjugation technologies. We have many years of research experience on liposome coupled antibodies or antibody fragments, including monoclonal antibody conjugations or antibody small fragment conjugations, providing many customers with targeted and stable immunoliposomes.
- Strategies combining stimulus responsive methods with immune targeting. We have developed a unique platform for the construction of intelligent multifunctional immunoliposomes, which has yielded numerous immunoliposomes capable of triggered drug release. The distinct features of the tumor microenvironment can act as endogenous stimuli, such as variations in pH, temperature, and enzyme levels, while exogenous stimuli like magnetic fields, light, heat, or ultrasound can also be utilized.
- Skilled teams. The research team's experts consist of scientists from various disciplines, with expertise in chemical synthesis, immunology, pharmaceutical formulations, and extensive field experience.
Published Data
Technology: Cationic liposomes grafted with trastuzumab technique
Journal: Pharmaceutics
IF: 5.4
Published: 2020
Results: In this study, the authors designed and developed PEGylated liposomes and Her2-targeted immunoliposomes. The average diameter is less than 200 nanometers, thus ensuring appropriate enhancement of permeability and retention (EPR) effects. The encapsulation rate of ASO is approximately 40%. Using the human PC-3 prostate cancer cell line as a standard model, the in vitro efficacy of free ASO and ASO encapsulated in liposomes or anti-Her2 immunoliposomes was tested in 2D and 3D spherical models. This preliminary study suggests that ASO can be encapsulated in liposomes and exert an antiproliferative effect on PCa. It is noteworthy that, although the expression of HER2 in the PC-3 model is weak, the use of surface mAb as a targeting agent can further improve efficacy, especially in prolonged exposure. Overall, the development of the third-generation ASO-iLi is helpful in utilizing the expression of HER2 in prostate cancer cells to achieve more precise in vivo effects and thereby improve efficacy.
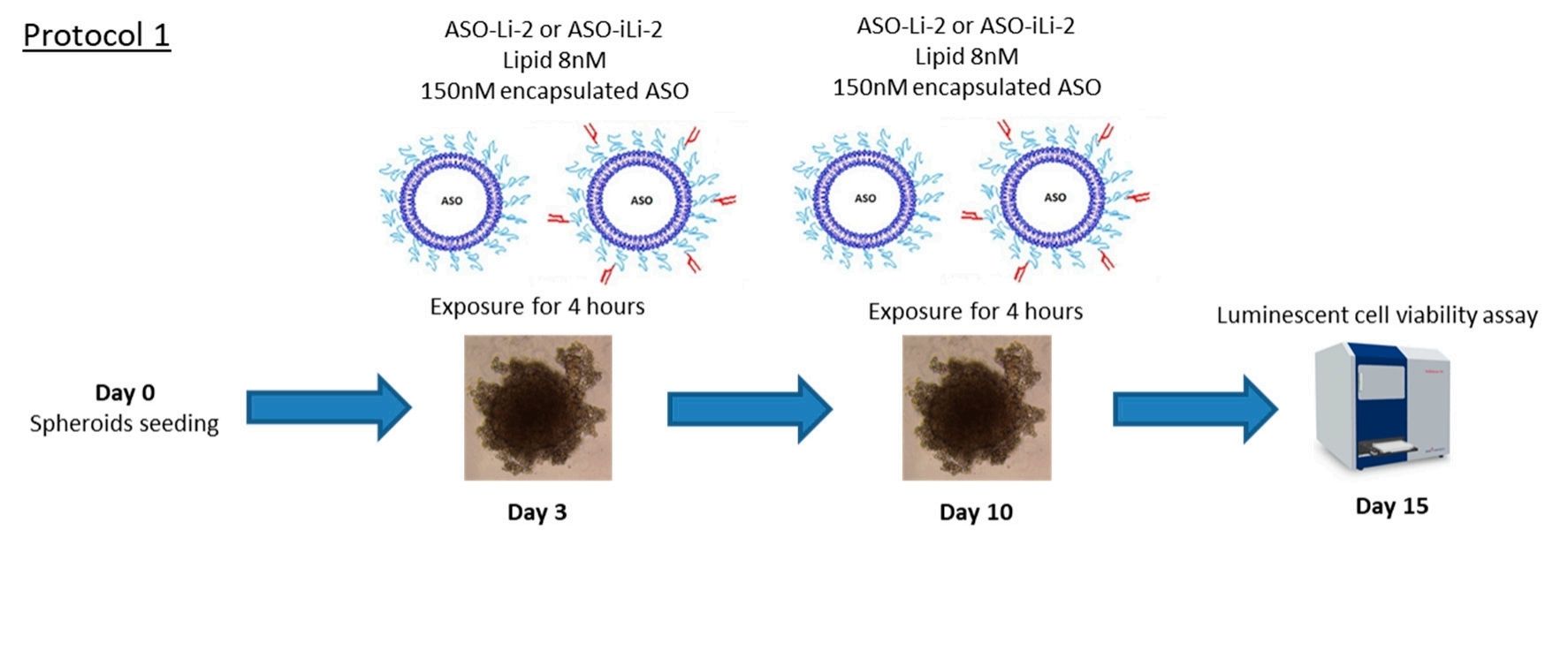 Fig.2 Scheme illustration of liposomal antiproliferative activity with short exposition time. (Sicard, Guillaume, 2020)
Fig.2 Scheme illustration of liposomal antiproliferative activity with short exposition time. (Sicard, Guillaume, 2020)
As a global nanoparticle development company, CD Formulation has extensive experience in the development and customization of immunoliposome services. If you need any help, please do not hesitate to contact us.
References
- Sicard, Guillaume., Paris, Clément, et al. Enhanced Antisense Oligonucleotide Delivery Using Cationic Liposomes Grafted with Trastuzumab: A Proof-of-Concept Study in Prostate Cancer. Pharmaceutics. 2020, 12. 1166.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 Schematic representation of the immunoliposome structure and composition. (Sicard, Guillaume., et al., 2020)
Fig.1 Schematic representation of the immunoliposome structure and composition. (Sicard, Guillaume., et al., 2020) Fig.2 Scheme illustration of liposomal antiproliferative activity with short exposition time. (Sicard, Guillaume, 2020)
Fig.2 Scheme illustration of liposomal antiproliferative activity with short exposition time. (Sicard, Guillaume, 2020)
