Custom Hollow Fiber Liposome Service
Inquiry
The technology for hollow fiber membrane contactors offers a novel method for developing liposomes, ideal for drug and cosmetic agent encapsulation. Known for its simplicity, speed, and suitability for large-scale continuous production of nanoliposome suspensions, this approach stands out. CD Formulation is proficient in hollow fiber technology and is committed to developing more advanced liposome products.
What are Hollow Fiber Liposomes?
Several techniques are available for producing liposome-based injectables in a GMP-compliant setting. The GMP production process typically involves liposome formation, size homogenization, organic solvent removal, liposome concentration control, and sterilization. However, ensuring scalability, reproducibility, and sterility for these complex and distinct processes can be challenging. To address these limitations, attention has turned to the combination of hollow fiber technology and liposome technology--hollow fiber liposome.
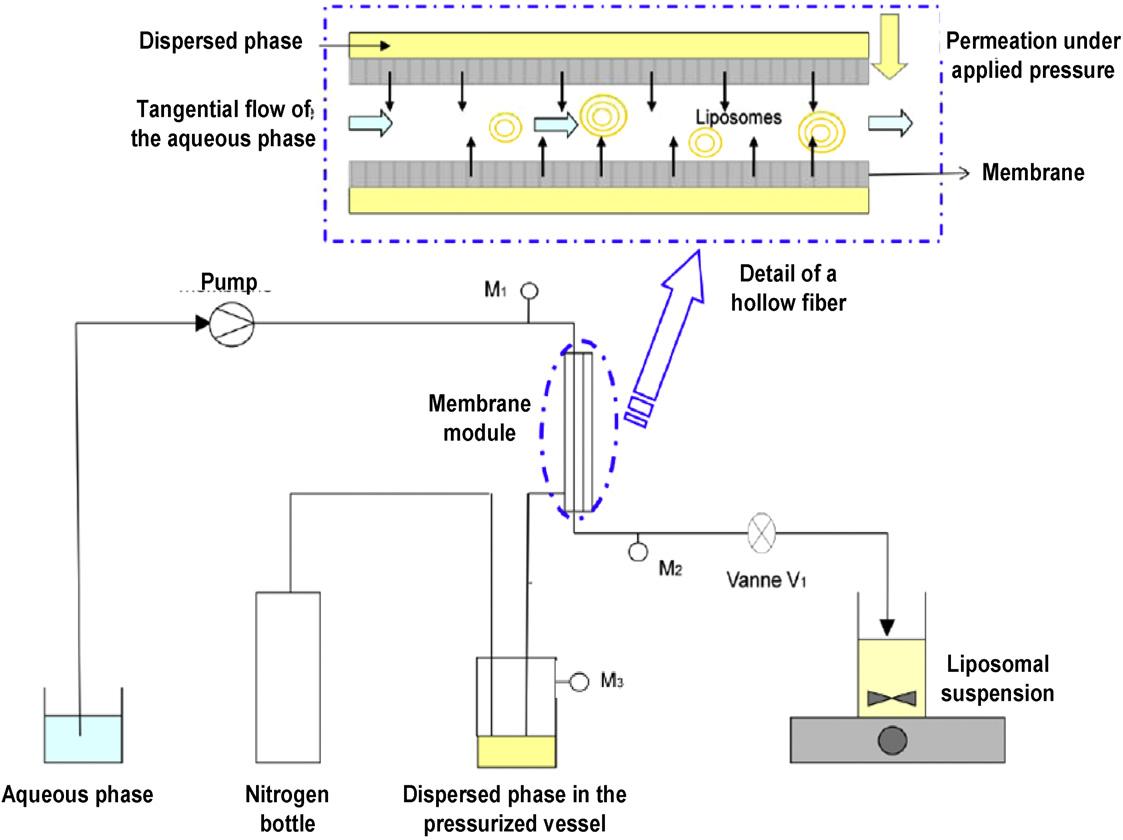 Fig.1 Preparation of liposomes using a membrane contactor. (Laouini A, et al., 2011)
Fig.1 Preparation of liposomes using a membrane contactor. (Laouini A, et al., 2011)
Our Hollow Fiber Liposome Customization Service
Hollow fiber structure study service
We are engaged in researching the composition of hollow fiber structures. The chemical properties and structure of hollow fiber membranes play a crucial role in separation performance, with key indicators including excellent film-forming properties, thermal stability, chemical resistance to acids and alkalis, as well as oxidation resistance.
Liposome characterization service
To evaluate the quality of liposomes and obtain quantitative measurements for batch-to-batch comparisons, we provide an evaluation of a variety of parameters, including average size and polydispersity index analysis, zeta potential determination, microscopy, encapsulation efficiency determination, and drug release studies.
Hollow fiber liposome microscreening services
Liposome hollow fibers can serve as a valuable screening tool for selecting natural active substances, especially in the initial screening and analysis of active compounds in natural herbal extracts. This capability allows us to identify permeable compounds that specifically bind to liposomes within the pores and tubes of the hollow fibers from natural herbal extracts.
Our Platforms for Hollow Fiber Liposome Customization
| Techniques and Platforms |
Specifics |
| Hollow fiber ultrafiltration membrane technique |
- A widely utilized ultrafiltration/diafiltration technology.
- The sample liquid flows in a gentle laminar state within the hollow fiber filtration assembly.
- To minimize shear stress on the target product in the liquid.
- Maintaining the structural and functional integrity of the product, which is particularly crucial for shear-sensitive products such as liposome-based formulations.
|
| Characterization techniques |
- Average size
- Polydispersity index analysis
- Zeta potential determination
- Microscopy
- Encapsulation efficiency determination
- Drug release
|
Why Choose CD Formulation?
- Hollow fiber ultrafiltration technique. This technique facilitates the advancement of innovative hollow fiber liposome products, offering customers comprehensive tailored services including formulation development and parameter optimization.
- Extensive and versatile applications of the products. Our products are widely applicable in various fields, including filtration and screening, cell culture, microbial collection, and more.
- The proficient teams. The technical team comprises seasoned and multidisciplinary experts specializing in hollow fiber liposome technology, possessing extensive knowledge in this domain.
Published Data
Technology: hollow fiber liposomes
Journal: Int J Pharms
IF: 5.8
Published: 2011
Results: In this study, the authors propose an innovative liposome preparation technique suitable for encapsulating pharmaceutical and cosmetic drugs. The method utilizes a membrane contactor within a hollow fiber structure. To investigate the process, the impact of key parameters on liposome characteristics was examined. The findings indicate that as organic phase pressure decreases, vesicle size distribution decreases, phospholipid concentration decreases, and the volume ratio of aqueous phase to organic phase increases. The liposomes are loaded with spironolactone, a hydrophobic drug model suitable for pediatric medicine. Transmission electron microscopy images reveal nanosized and spherical multilayered vesicles. The release profile demonstrates rapid and complete release within approximately 5 hours.
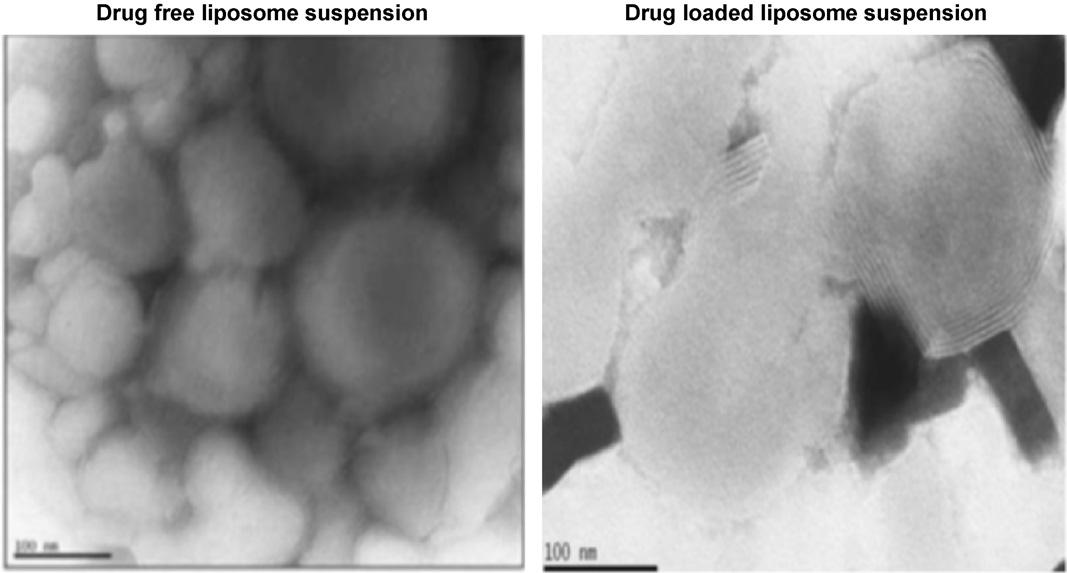 Fig.2 TEM micrographs of drug-free and drug-loaded liposome suspension. (Laouini A, et al., 2011)
Fig.2 TEM micrographs of drug-free and drug-loaded liposome suspension. (Laouini A, et al., 2011)
As a leading company in nanoparticle development, CD Formulation is dedicated to providing excellent hollow fiber liposome products. Please do not hesitate to contact us if you require any assistance.
References
-
Laouini A, Jaafar-Maalej C, et al. Liposome preparation using a hollow fiber membrane contactor--application to spironolactone encapsulation. Int J Pharm. 2011; 415(1-2): 53-61.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Preparation of liposomes using a membrane contactor. (Laouini A, et al., 2011)
Fig.1 Preparation of liposomes using a membrane contactor. (Laouini A, et al., 2011) Fig.2 TEM micrographs of drug-free and drug-loaded liposome suspension. (Laouini A, et al., 2011)
Fig.2 TEM micrographs of drug-free and drug-loaded liposome suspension. (Laouini A, et al., 2011)
