Custom Glucose Modified Liposome (GML) Service
Inquiry
Glucose transporters on the surface of the cell membrane can specifically bind and transport glucose, galactose, mannose, and its derivatives, and this process can fully provide ATP and energy required by cancer cells. Therefore, sugars as modifiers can greatly improve the cancer targeting of liposomes. CD Formulation has built our glycosylation modification platform for liposomes, aiming to provide customized glycosyl-modified liposomes including glucose modified-liposomes with high quality and stability.
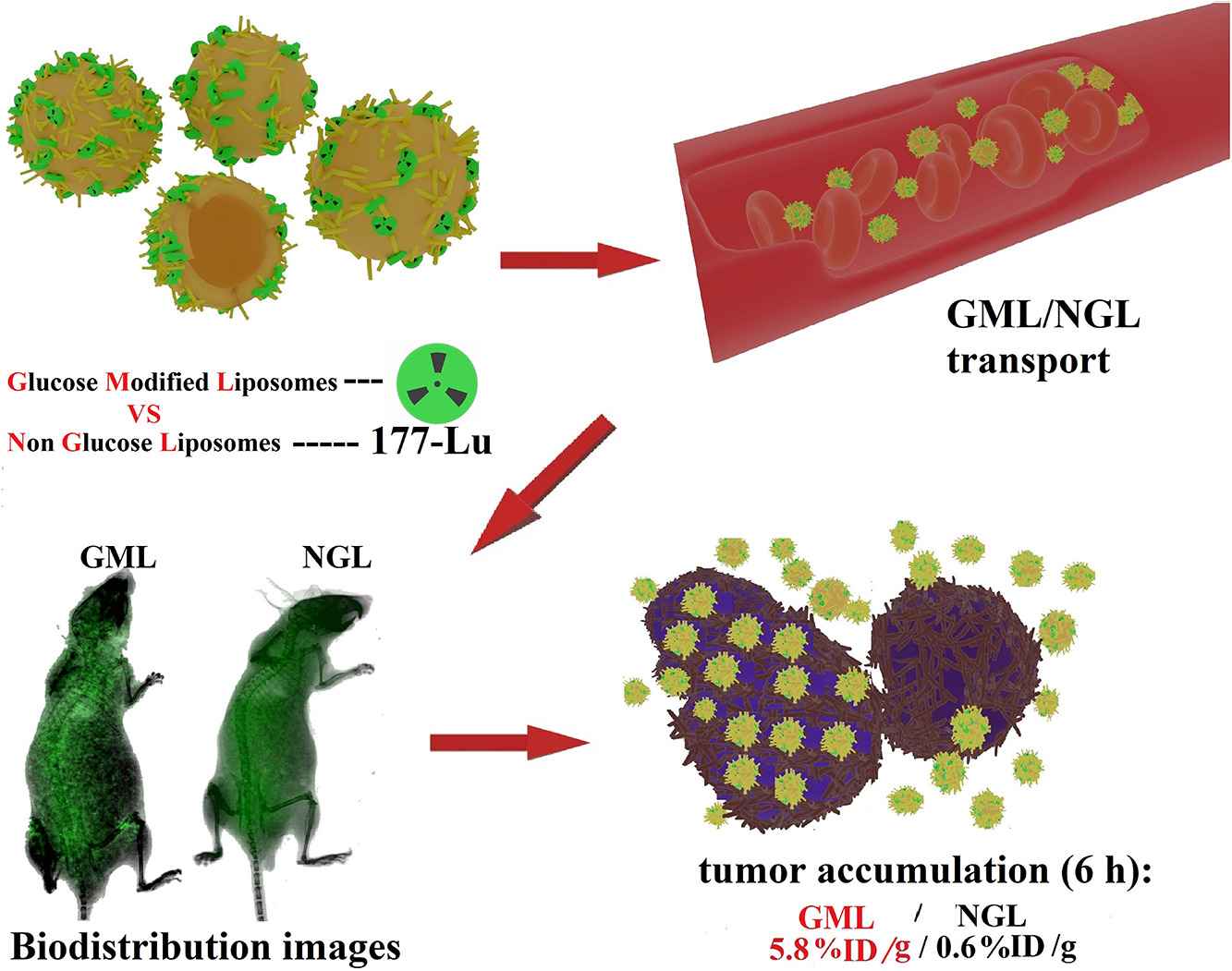 Fig.1 Schematic diagram of glucose-modified liposomes. (Đorđe Cvjetinović, et al., 2021)
Fig.1 Schematic diagram of glucose-modified liposomes. (Đorđe Cvjetinović, et al., 2021)
What Are Glucose Modified Liposomes?
Sugar is the main source of energy for cells, and cells produce ATP for energy through glycolysis. Compared to normal cells, tumor cells lose the ability to control their growth and therefore need to ingest large amounts of ATP to survive. This requirement is met by increased expression of the glucose transporter (glut) on the surface of the cell membrane. It can also transport galactose, mannose, and their derivatives. According to the characteristics of different cells, a variety of glucose receptors are also expressed on the cell surface, which indicates that glycol groups can be used as ligands to modify liposomes and achieve the purpose of targeting tumor cells. Although normal cell surfaces also express GLUT to take up glucose, due to the tumor's loss of growth control and great demand for energy, the tumor cell surface will overexpress GLUT to maintain its energy requirements. Glucose-modified liposomes use overexpressed GLUTs on the surface of tumor cells as targets to achieve precise targeted drug delivery to tumor cells.
Explore Our Custom Glucose Modified Liposome Service
Liposome Glycosyl Modification
We can offer a wide range of glyco-based modification/customization services. In addition to liposomes modified by glucose/glucose derivatives, we offer liposomes modified by galactose/galactose derivatives, liposomes modified by mannose/mannose derivatives, etc.
Liposome Characterization
This service is designed to provide physicochemical properties analysis services for customized liposomes, including but not limited to particle size analysis, potential distribution analysis, structural analysis, physicochemical stability body analysis, encapsulation rate, drug loading, etc.
In Vitro Stability and Receptor Binding Studies
In vitro evaluation is the link between in vivo evaluation of products. Therefore, we also offer various in vitro evaluation services such as in vitro stability, tumor accumulation and glucose uptake rate of tumor cells, Ex vivo biodistribution studies, etc.
In Vivo Evaluation
This service is designed to provide in vivo release, targeting analysis, off-target effect analysis, in vivo distribution characteristics, stability, and a spectrum of characterization services for customized liposomes.
Our Capabilities for Customizing Glucose Modified Liposomes
The platforms and technologies we can offer for custom GML services are summarized below.
| Items |
Detailed Information |
| Radiotracer techniques |
- Radiotracer technique is a reliable and precise method for in vitro and in vivo biodistribution studies of newly developed nanostructures.
|
| In vivo evaluation and imaging techniques |
Liposome in vivo evaluation system:
- Fluorescence imaging analysis
- Various in vivo evaluation models are available: the leukemia models represented by P388 and L1210, Lewis lung cancer mouse model, B16 mouse model, nude mouse breast cancer transplant tumor model, human renal clear cell adenocarcinoma metastasis model in nude mice, etc.
|
| In vitro models |
- Infiltrating glioma models
- Animal models of inflammatory bowel disease (IBD)
- Rheumatoid arthritis (RA) animal models
- Encephalomyelitis (EAE) animal models
|
Our Key Advantages in Customizing Glucose Modified Liposomes
- Strong R&D team. The team consists of a considerable multitude of scientists and experts who are proficient in the development, analysis, in vivo and in vitro characterization of various glycosyl-modified liposomes.
- Comprehensive characterization platforms. Our in vivo and in vitro characterization platform, developed primarily for glucose-modified liposomes, can provide a variety of disease model systems to characterize customized product stability and bioactivity in vivo and in vitro. Our physicochemical platform mainly helps customers analyze the physicochemical properties of customized liposomes.
- Quality service. Each project will be carefully communicated with the customer before the start of the project details, each project node stage will have a project summary report, designed to help customers understand project progress.
Published Data
Technology: Glucose-modified liposomes technique in brain delivery
Journal: International Journal of Nanomedicine.
IF: 5.5
Published: 2012
Results: In this study, coumarin-6 liposomes (GLU200-LIP, GLU400-LIP, GLU1000-LIP, and GLU2000-LIP) composed of phospholipids and glucose-derived cholesterol were prepared by thin film dispersive-ultrasound method. The in vitro blood-brain barrier model was established to evaluate the ability of different liposomes to cross the blood-brain barrier. In vitro and in vivo near-infrared fluorescence imaging and confocal laser scanning microscopy were used to identify the biological distribution of liposomes in the rat brain, and quantitative analysis was performed by high-performance liquid chromatography. The data indicated that GLU400-LIP, GLU1000-LIP, and GLU2000-LIP all had brain-targeting potential, with GLU1000-LIP showing the strongest brain delivery capability as a promising drug delivery system.
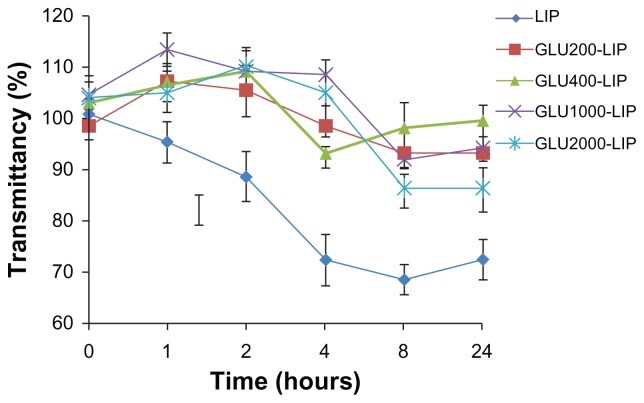 Fig.2 The variation in transmittancy versus the different incubation time of glucose-liposomes. (Xie, F., et al., 2012)
Fig.2 The variation in transmittancy versus the different incubation time of glucose-liposomes. (Xie, F., et al., 2012)
CD Formulation is available in a variety of glucose-modified liposome customization services. If you need any help, please do not hesitate to contact us.
References
- Đorđe Cvjetinović, Željko Prijović, et al. Bioevaluation of glucose-modified liposomes as a potential drug delivery system for cancer treatment using 177-Lu radiotracking. Journal of Controlled Release, 2021, Volume 332, Pages 301-311.
- Xie, F., Yao, N., et al. Investigation of glucose-modified liposomes using polyethylene glycols with different chain lengths as the linkers for brain targeting. International Journal of Nanomedicine. 2012, 7, 163–175.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services

 Fig.1 Schematic diagram of glucose-modified liposomes. (Đorđe Cvjetinović, et al., 2021)
Fig.1 Schematic diagram of glucose-modified liposomes. (Đorđe Cvjetinović, et al., 2021) Fig.2 The variation in transmittancy versus the different incubation time of glucose-liposomes. (Xie, F., et al., 2012)
Fig.2 The variation in transmittancy versus the different incubation time of glucose-liposomes. (Xie, F., et al., 2012)
