Custom Dual Redox-responsive Liposome Service
Inquiry
Stimulus-responsive delivery systems hold great promise in cancer treatment. Recently, dual redox-responsive liposomes have garnered significant attention as a novel stimulus-responsive delivery method. CD Formulation is dedicated to leveraging state-of-the-art technologies and specialized expertise to create high-performance liposomes that respond to dual redox conditions, providing advanced solutions for precise and effective drug delivery.
Mechanism of Dual Redox-responsive Liposomes
Redox signaling plays an important role in many aspects of physiology, including the cardiovascular system. Disorder of redox regulation is associated with many pathological conditions. Redox signaling involves the production of a particular redox species in a specific place at a specific time. In the tumor microenvironment, cancer cells have higher levels of glutathione (about 2-20 mM) due to specific environmental and nutritional requirements as well as genetic changes. When administered, nanocarriers can accumulate in tumor tissues over long cycles by enhancing permeability and retention (EPR) and be absorbed by tumor cells through endocytosis. Subsequently, at high redox potential, the redox reaction chain is destroyed, resulting in structural decomposition of the nanocarriers leading to rapid drug release. In healthy tissues with low redox potential, the nanocarrier structure remains intact by exploiting the reduction potential gradient between cancer cells and healthy cells, minimizing side effects. Dual redox-responsive liposomes represent an innovative multi-reactive liposome system.
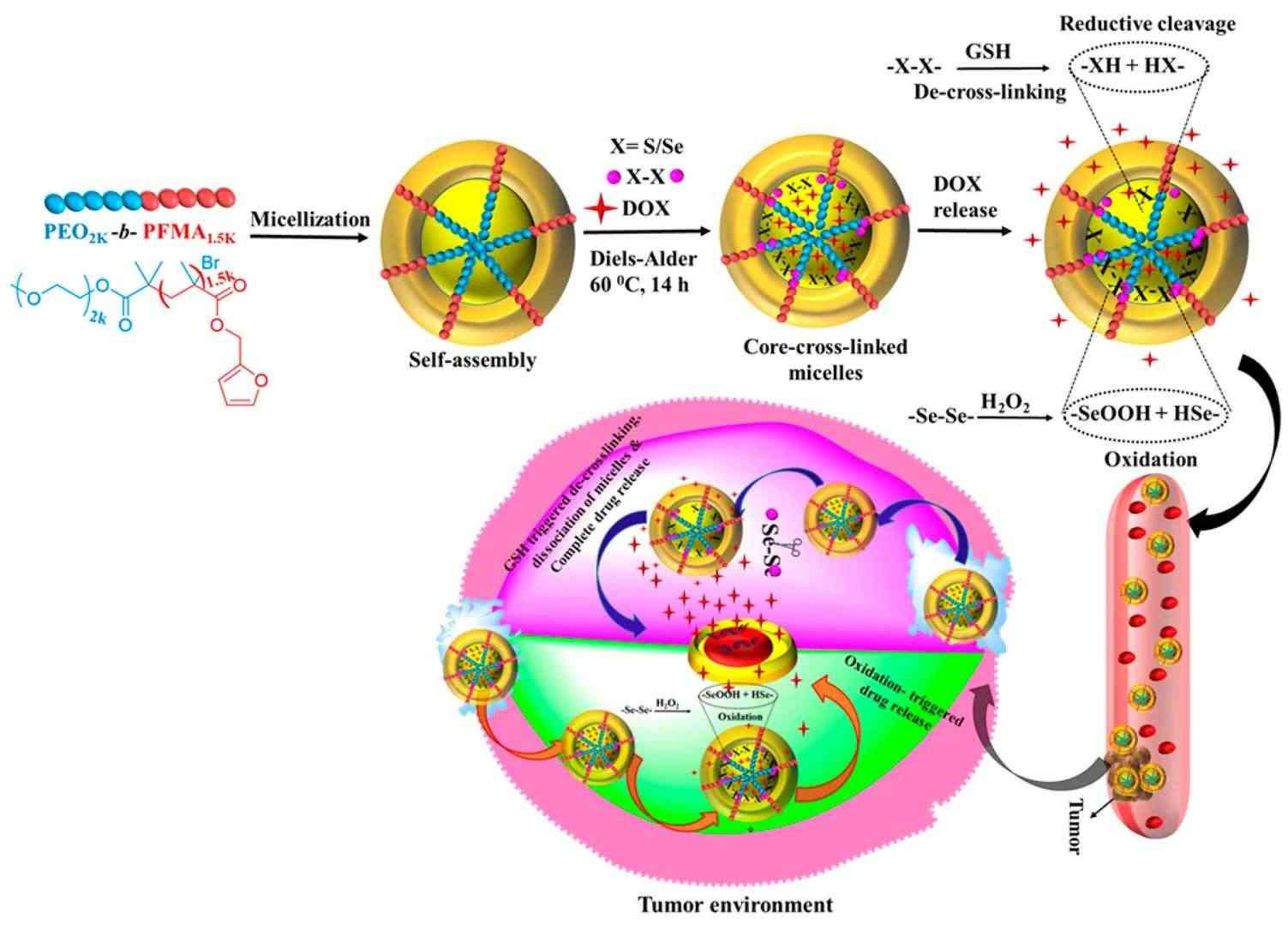 Fig.1 Schematic representation of DOX release under the conditions of GSH and H2O2. (Yadav S, et al., 2023)
Fig.1 Schematic representation of DOX release under the conditions of GSH and H2O2. (Yadav S, et al., 2023)
Our Dual Redox-responsive Liposome Customization Service
Screening of redox types and trigger strategies
Redox systems are crucial for maintaining cell homeostasis. In physiological conditions, cells maintain oxidation-reduction balance by generating and eliminating reactive oxygen/nitrogen species (ROS/RNS). We help our clients build suitable trigger strategies by studying ROS systems (such as free radicals like superoxide (O2−) and hydroxyl radical (HO·), as well as non-free radicals like hydrogen peroxide (H2O2)) and RNS systems (including nitric oxide (NO·), peroxynitrite (ONOO−)), GSH system, thioredoxin (Trx) system, glutaredoxin (Grx) system, and peroxidases (Prxs).
Synthesis of crosslinking agents
Crosslinkers play a crucial role in the development of stimulation-responsive vectors, and disulfide/diselenide bonds are a good candidate for crosslinkers because of their low bond dissociation energy and susceptibility to oxidation even under weak stimuli. To help customize dual oxidation-reduction liposomes that meet specific requirements, we use screening and a combination of linkers.
Study of Redox-response characteristics
The service focuses on studying the Redox response behavior of liposomes by observing their morphology and size changes in different Redox environments. We will first simulate the conditions surrounding the tumor cells (GSH and H2O2 environment) and then observe the structural changes during lipid incubation with GSH and H2O2.
Characterization analysis
We provide physical and chemical characterization of liposomes such as particle size, charge distribution, charge load and encapsulation rate, viscosity, rheology, appearance, stability, etc. In vitro characterization services include cell uptake rate assay, cytotoxicity assay, and in vitro release assay. In vivo characterization services such as in vivo distribution analysis, in vivo bioactivity analysis, in vivo stability analysis, etc.
Our Platforms for Dual Redox-responsive Liposome Customization
| Techniques and Platforms |
Specifics |
| Redox trigger strategies study platform |
- To study the trigger mechanism of redox stimuli.
- Study on the various types of redox-triggered mechanisms.
|
| Synthesis techniques of crosslinking agents |
- To develop novel crosslinking agents.
- Synthesis of redox crosslinkers.
|
| Characterization Platform |
- Physical and chemical characterization.
- In vitro and in vivo characterization.
- Advanced analytical instruments for in vitro and in vivo analysis.
|
Why Choose CD Formulation?
- Intelligent dual redox responsive delivery system development platform. The platform supports the development of innovative dual redox-responsive liposomes, from formulation development and process optimization to analytical characterization, aiming to provide highly efficient and intelligent dual-responsive liposomes.
- Innovative techniques. The systematic investigation of redox-triggering mechanism offers a pathway for novel types of double-redox responsive liposomes.
- The proficient teams. The technical teams are led by groups of seasoned experts with extensive knowledge and experience in redox-responsive liposomes, boasting diverse academic backgrounds across various disciplines.
- Advanced analytical equipment and platform. The platform is equipped with the most advanced analytical instruments for verifying physical and chemical indicators and conducting both in vitro and in vivo analyses for the qualities of customized products.
Published Data
Technology: PD-L1 inhibitor conjugate polymer-liposomes technique
Journal: ACS Applied Materials & Interfaces
IF: 9.5
Published: 2017
Results: By the non-covalent supramolecular inclusion body interaction between ferrocene (CPT) and β-cyclodextrin (β-CD) modified by camptothecin at the end of methoxy polyethylene glycol (mPEG), a double REDOX and biolever-triggered supramolecular system was established. Using these two fragments, it is possible to form a stable non-covalent supramolecular structure, namely mPEG-β-CD/Fc-CPT, which then self-assembles into a micellar structure in water. Interestingly, these supramolecular micelles exhibited uniform globular structure, high and constant drug loading, ultrafast REDOX reactive drug release, and showed the same cell proliferation inhibition against A549 cancer cells. Cytotoxicity evaluation of mPEG-β-CD also showed good biocompatibility. It is expected that this supramolecular complex may become an alternative to a new promising drug delivery system.
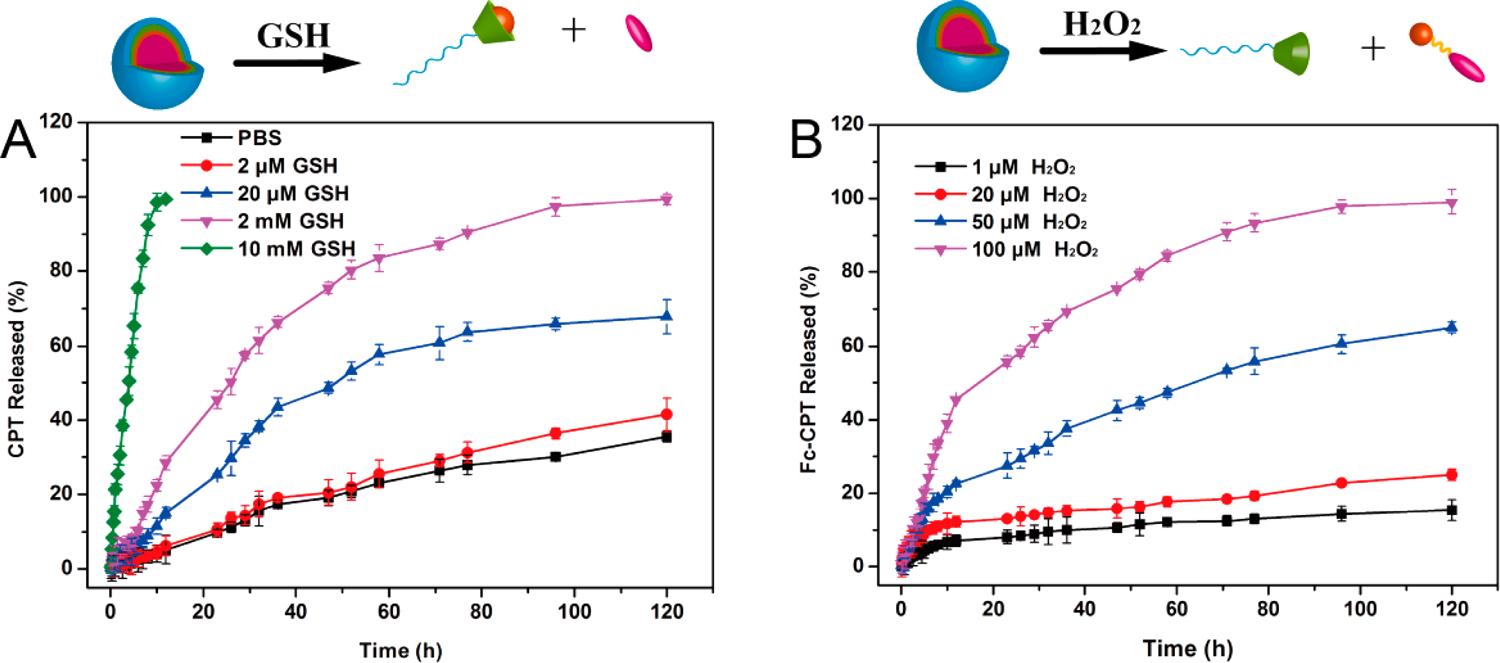 Fig.2 Release profiles of CPT from the mPEG-β-CD/Fc-CPT supramolecular complex micelles. (Yang Kang, et al., 2017)
Fig.2 Release profiles of CPT from the mPEG-β-CD/Fc-CPT supramolecular complex micelles. (Yang Kang, et al., 2017)
With state-of-the-art technology and platforms, CD Formulation strives to deliver the most intelligent dual redox-responsive liposomes. If you require any assistance, please do not hesitate to contact us.
References
-
Yadav S, Ramesh K, et al. Redox-Responsive Comparison of Diselenide and Disulfide Core-Cross-Linked Micelles for Drug Delivery Application. Pharmaceutics. 2023, 15(4):1159.
- Yang Kang, Xin Ju, et al. Reactive Oxygen Species and Glutathione Dual Redox-Responsive Supramolecular Assemblies with Controllable Release Capability. ACS Applied Materials & Interfaces. 2017, 9(5), 4475-4484.
How It Works
STEP 2
We'll email you to provide your quote and confirm order details if applicable.
STEP 3
Execute the project with real-time communication, and deliver the final report promptly.
Related Services


 Fig.1 Schematic representation of DOX release under the conditions of GSH and H2O2. (Yadav S, et al., 2023)
Fig.1 Schematic representation of DOX release under the conditions of GSH and H2O2. (Yadav S, et al., 2023) Fig.2 Release profiles of CPT from the mPEG-β-CD/Fc-CPT supramolecular complex micelles. (Yang Kang, et al., 2017)
Fig.2 Release profiles of CPT from the mPEG-β-CD/Fc-CPT supramolecular complex micelles. (Yang Kang, et al., 2017)
