Replication Competent Lentivirus Testing
Inquiry
Viral vectors have an important role in gene therapy, however, in the development and production of gene therapy formulations, there may be problems with the emergence or presence of replication-competent lentiviruses (RCLs) or replication-competent retroviruses (RCRs) in the preparation of gene therapy vectors, which can affect the safety of gene therapy formulations. Therefore, testing for replication-competent viruses that may be generated by recombination at various stages of vector preparation is a key factor in the manufacture of these vectors.
CD Formulation provides comprehensive technical support services for the development of gene therapy formulations, particularly for the testing of replicable viruses for viral vector-based gene therapy programs. By understanding the issues faced by researchers in the development of gene therapy formulations, we continue to provide high-quality, reliable testing services to ensure the safety of the gene therapy formulations being developed.
The Importance of Replication Competent Lentivirus Testing
Replication-competent lentivirus (RCL) detection plays a vital role in the quality control of gene therapy formulations and is a key component in ensuring product safety, efficacy and compliance. Detection of RCL is of great importance for quality control of gene therapy formulations, mainly in the following aspects.
- Safety assurance. Testing RCL can ensure that gene therapy products do not contain viruses capable of replication and transmission, thus guaranteeing the safety of the treatment. RCL testing is one of the key indicators for quality control of gene therapy formulations.
- Regulatory compliance. As gene therapy technology has evolved, regulatory agencies worldwide have issued guidelines for gene therapy products that include specific requirements for RCL testing.
- Risk assessment and management. RCL testing can help researchers assess and control the risks that may arise during the production of gene therapy formulations, including potential contamination and instability of viral vectors.
- Promote the development of the industry. Uniform and standardized RCL assays can help improve the quality control level of the whole industry and promote the healthy development of gene therapy formulations.
Explore Our Replication Competent Lentivirus Testing
The purpose of the RCL assay is to ensure that replication-competent viruses are not present in gene therapy preparations, thereby reducing the potential for adverse effects in patients receiving the therapy. We offer assays for RCL that include but are not limited to, indicated cell culture assays, ELISA (p24 protein assay), PCR/q-PCR, and transcriptase activity determination (PERT). The selection and application of these methods need to be determined based on specific product characteristics and regulatory requirements. Below is a brief description of the methods we offer.
Indicator cell culture assay
This is a gold standard assay that determines the presence of RCL by co-culturing the sample to be tested with specific cells and then detecting viral proteins or viral genes in the culture medium.
ELISA (p24 protein assay)
This method is suitable for detecting viruses in which RCL is formed by recombination between viral genomes during vector production, but may not be applicable to all types of pseudotyped viruses.
PCR and q-PCR method
This method determines the presence of RCL by detecting specific viral gene sequences (e.g., VSV-G gene or psi-gag gene sequences), and has the advantages of high sensitivity and simplicity.
PERT method (transcriptase activity measurement)
This method detects RCL by measuring reverse transcriptase activity, but may have a high background in some cell assays.
Our Process of Replication Competent Lentivirus Testing
The RCL testing experimental process typically includes the following key steps.
Sample preparation
Selection of appropriate samples for testing, which may include untreated viral supernatant, concentrated or purified viral supernatant, or finished virus.
Cell culture
Using a sensitive indicator cell line, the sample is co-cultured with the cells, which usually takes 21 days with at least 5 passages.
Endpoint testing
At the end of the culture, endpoint assays are performed using a variety of methods to ensure the accuracy of the results. These methods may include ELISA, qPCR/PCR, and PERT.
Data analysis
Assay results are analyzed for the presence of RCL.
Validation and reporting
Validation of the assay to ensure the reliability and reproducibility of the method.
Records and tracking
Detailed records of experimental procedures and results, including the setting of positive and negative controls, as well as the management of samples, to ensure data traceability.
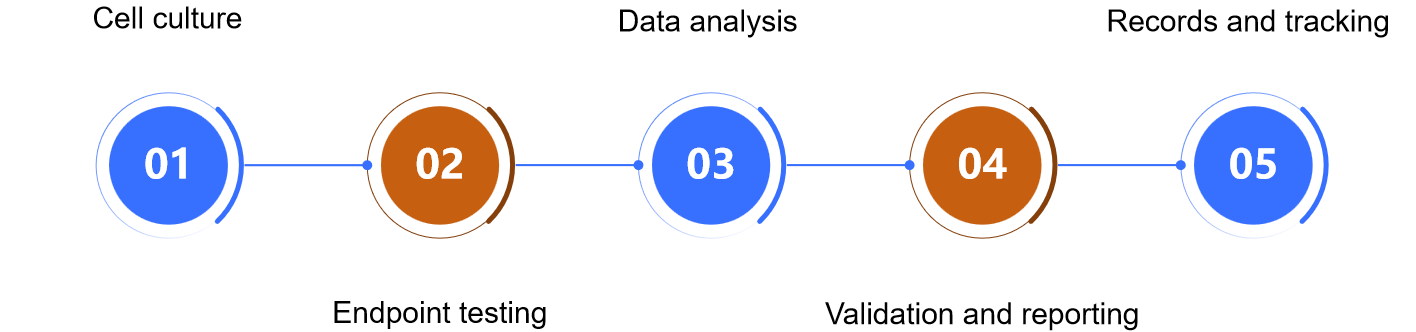 Fig.1 The process of replication-competent lentivirus testing. (CD Formulation)
Fig.1 The process of replication-competent lentivirus testing. (CD Formulation)
Our Equipment and Instruments for Replication Competent Lentivirus Testing
| Equipment and Instruments |
| Cell culture equipment |
ELISA reader |
PCR instrument |
| Nucleic acid extraction equipment |
Digital PCR |
Real-time PCR |
| Flow cytometer |
Learn more |
Highlights of Our Replication Competent Lentivirus Testing
- Professional technical team. We have a team of experts in the fields of molecular biology, virology, cell biology and bioinformatics to ensure the accuracy and reliability of the test.
- Advanced equipment. We are equipped with a real-time quantitative PCR system, digital PCR system, automated equipment for nucleic acid extraction, enzyme labeling instrument and other advanced instruments to support high-precision testing.
- Standardized operating procedures. We follow international standards and guidelines to ensure standardization and normalization of the testing process.
- Comprehensive services. We provide one-stop services from sample collection, nucleic acid extraction, testing to result analysis to meet customers' needs at different stages.
Published Data
Technology: Replication competent lentivirus testing
Journal: Mol Ther
IF: 12.4
Published: 2018
This study evaluated T-cell products from 26 clinical trials for replication-competent lentivirus (RCL), a theoretical safety concern in lentiviral gene therapy. Using methods from the National Gene Vector Biorepository, researchers tested 460 cell products from 375 subjects. All samples were RCL-negative, and no RCL infection was detected in 296 participants screened post-infusion. The results support the safety of third-generation lentiviral vectors and suggest that testing T-cell products for RCL may not offer additional benefits beyond screening the lentiviral vector itself.
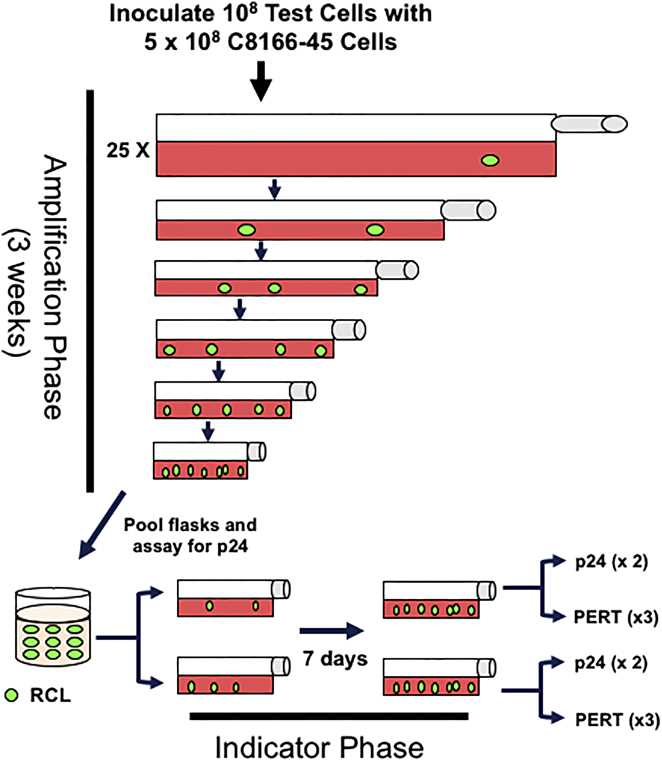 Fig.2 Schematic representation of the replication-competent lentivirus assay. (Cornetta K, et al., 2018)
Fig.2 Schematic representation of the replication-competent lentivirus assay. (Cornetta K, et al., 2018)
CD Formulation offers a comprehensive range of testing services in the quality control of gene therapy formulations. We can provide customized solutions based on the individual needs of our clients in terms of science, timeline and cost to best meet their requirements. If you are interested in us, please feel free to contact us.
References
- Cornetta K, et al. Absence of Replication-Competent Lentivirus in the Clinic: Analysis of Infused T Cell Products. Mol Ther. 2018, 26(1):280-288.
Related Services

 Fig.1 The process of replication-competent lentivirus testing. (CD Formulation)
Fig.1 The process of replication-competent lentivirus testing. (CD Formulation) Fig.2 Schematic representation of the replication-competent lentivirus assay. (Cornetta K, et al., 2018)
Fig.2 Schematic representation of the replication-competent lentivirus assay. (Cornetta K, et al., 2018)