Plasmid DNA Production for Gene Therapy
Inquiry
Plasmids of high-quality DNA it is a key component in the production of gene therapy formulations and are therefore in high demand. Therefore, it is very necessary to optimize the production to meet the quantitative and qualitative requirements for the production of gene therapy formulations. Plasmid DNA (pDNA) has some challenges in production due to its large size, high cleavage sensitivity, high viscosity, and its similarity to impurities in the production process, etc. CD Formulation has continuously optimized its technology system based on the challenges of plasmid DNA production, and perfected and optimized the production of plasmid DNA, which assists customers in gene therapy GMP production.
The Importance of Plasmid DNA Production for Gene Therapy
Plasmid DNA plays an indispensable role in gene therapy. It not only carries exogenous target sequences to facilitate the development of therapeutic agents but also constitutes a central component of cellular and genetic material processing programs. The abundance and purity of plasmid DNA are directly related to the efficacy and safety of gene therapy formulations, and therefore, strict quality standards must be followed during their manufacture.
- Gene transfer vector. Plasmid DNA is responsible for introducing the desired coding fragments into specific types of cells in the host to achieve the desired experimental purpose. Designing the vector structure and optimizing the expression conditions, can help researchers to significantly increase the success rate of transgenic technology, which in turn can facilitate the development of gene therapy formulations.
- Basis for vaccine development. Some gene vaccines encode antigenic proteins through plasmid DNA to stimulate the body's immune response.
- Stabilization of production gene expression. The structure and design of plasmids directly affect the efficiency and persistence of gene expression in vivo, so high-quality plasmid production is needed to ensure the stability of gene expression.

Explore Our Plasmid DNA Production for Gene Therapy
Plasmid strain construction to GMP production
Our plasmid DNA production involves a full range of integrated services from plasmid strain construction, clone screening, strain banking, process development, non-clinical research grade plasmid production to GMP grade plasmid production. We can provide high-quality, customized plasmid products to accelerate our customers' product development and application supply.
Upstream process optimization
The upstream process includes the selection of host cell lines, optimization of fermentation conditions and plasmid copy number control. Optimization of host cell line selection can improve the replication ability and stability of plasmids. Optimization of fermentation conditions can significantly increase pDNA yield while maintaining superhelical DNA content. Copy number control also has an important impact on the yield and quality of pDNA.
Cell lysis and neutralization
Cell lysis is a critical step in the production of plasmid DNA and is usually performed by alkaline lysis, including in combination with detergents. Lysis incubation time affects the quality and quantity of pDNA, which we optimize to ensure pDNA quality. The molecular basis of alkali lysis is to irreversibly damage genomic DNA while leaving pDNA intact.
Chromatographic purification and concentration for sterilization
Chromatographic purification is used to separate plasmid DNA from other components of the host cell, a step that typically uses anion-exchange chromatography and hydrophobic interaction chromatography to remove impurities such as low molecular weight host RNA. Subsequently, the purified pDNA is formulated with excipients and then filtered through a sterilizing filter.
Quality control (QC)
Each step of plasmid DNA production includes comprehensive process control and quality testing. Our quality control includes strict control of bacterial endotoxin, host bacterial protein residues, exogenous DNA residues and residual RNA.
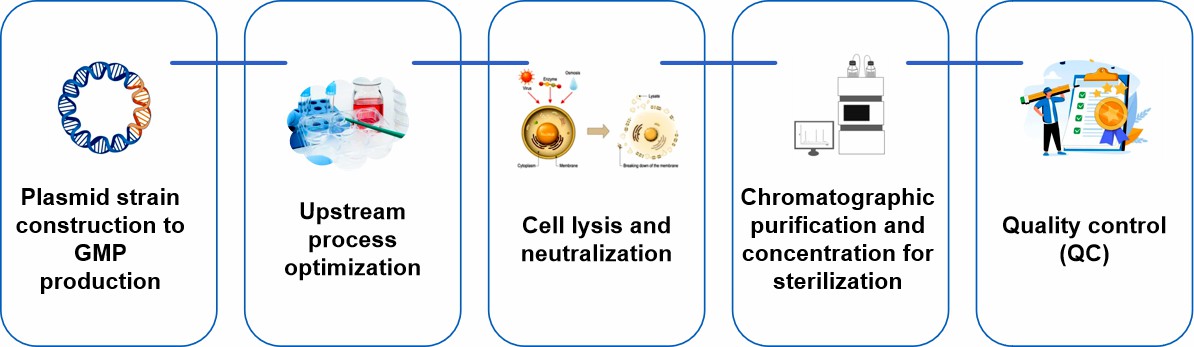 Fig.2 Our process of plasmid DNA production. (CD Formulation)
Fig.2 Our process of plasmid DNA production. (CD Formulation)
Our Technologies for Plasmid DNA Production
| Platforms & Technologies |
Content Description |
| High density fermentation system |
We use high-density fermentation technology to improve the yield and quality of plasmids, while increasing production efficiency through optimized nutrient supply and control parameters. |
| Chromatographic purification technology |
We utilize purification processes such as ion exchange, hydrophobic or affinity chromatography to effectively remove impurities and improve plasmid purity and stability. |
| Endotoxin removal technology |
We strictly control endotoxin content through a multi-step purification process to ensure that the final product meets high quality standards. |
| Quality control platform |
We have a robust quality control platform, which utilizes qPCR, restriction enzyme digestion, gel electrophoresis and other assays to conduct comprehensive testing of plasmid DNA. |
Highlights of Plasmid DNA Production for Gene Therapy
- Comprehensive technical support. We provide integrated services from plasmid construction, strain library construction, process development to quality methodology research.
- Standard production platform. We have a GMP-compliant plasmid production platform to ensure product quality.
- Mature process development and production experience. We have mature process development and GMP production experience and have assisted many customers in completing plasmid DNA production projects with high efficiency.
- Multiple cooperation platforms. We have cooperated with top international research institutions to build a full chain of gene therapy formulation development and innovation platforms to accelerate the industrialization of gene therapy formulations.
Published Data
Technology: Plasmid DNA production
Journal: Acta Biochim Pol
Published: 2005
Gene therapy and gene vaccines promise to revolutionize the treatment of genetic and acquired diseases. Since viral vectors typically have many drawbacks when applied to humans, administration of naked DNA or DNA packaged in liposomes or polymer complexes becomes a viable alternative. In this article, the authors, in response to the growing demand for pharmaceutical-grade plasmids, have developed a novel and economical downstream process that overcomes the bottlenecks of common laboratory-scale techniques and meets all regulatory requirements. After cell lysis by a gentle, automated continuous system developed in-house, a sequence of hydrophobic interaction, anion exchange and size exclusion chromatography ensure the separation of impurities as well as unwanted plasmid isomers. Concentration adjustment and final filtration followed the sequential chromatographic steps. This process ultimately provides high-purity plasmid DNA with up to 98% homogeneity in the superhelical form in high yields in any desired final buffer.
CD Formulation is committed to providing efficient and professional plasmid DNA production services to support the development and innovation of gene therapy formulations. We help our clients achieve breakthroughs in the development of gene therapy formulations by continuously optimizing our technology and improving our service quality. If you are interested in us, please feel free to contact us.
Reference
- Urthaler J, et al. Improved downstream process for the production of plasmid DNA for gene therapy. Acta Biochim Pol. 2005, 52(3):703-11.


 Fig.2 Our process of plasmid DNA production. (CD Formulation)
Fig.2 Our process of plasmid DNA production. (CD Formulation) 