Injectable Gene Therapy Formulation Development
Inquiry
Injectable gene therapy is a cutting-edge technology for treating or repairing hereditary and certain acquired diseases by introducing specific genes directly into the patient's body. Injectable gene therapy combines modern gene technology and delivery systems to provide revolutionary treatments, especially in the field of refractory and hereditary diseases. In-depth research on injectable gene therapy is of great significance in advancing the study of gene therapy drug delivery systems. Based on years of research experience and a professional drug development research team, CD Formulation can provide customers with efficient and reliable development services for gene therapy drug delivery systems, especially for the research and development of injectable gene therapy. Our technical support for injectable gene therapy will not only bring about new therapeutic approaches but also help researchers gain a deeper understanding of the role of genes in health and disease.
Advantages of Injectable Gene Therapy Formulation
- Lasting results. Injectable gene therapy is expected to provide long-term or even permanent therapeutic effects because it directly modifies or repairs the problem gene.
- High precision. Modern gene editing techniques such as CRISPR-Cas9 allow editing at the level of individual base pairs, which greatly improves the precision of treatment and reduces side effects in non-targeted gene regions.
- Single treatment. Many gene injectable therapies require only one treatment to see significant results, reducing the pain and financial burden of repeated treatments for patients.
- Wide applicability. This therapy is not limited to a single type of disease. Multiple diseases caused by genetic defects can be repaired with specific gene injectable therapy.

Our Services for Developing Injectable Gene Therapy Formulation
Preliminary research and discovery
Includes the selection and validation of gene targets and basic research on gene function. This phase focuses on gene identification and target validation.
Vector design and construction
Viral vectors such as adeno-associated virus, adenovirus, or retrovirus are usually used and the target gene is inserted into the vector DNA to ensure efficient expression in the host cell. This step requires optimization of the vector for efficient gene delivery and reduction of immune response.
In vitro studies and in vivo studies
The safety and efficacy of the vector are tested in cellular models. The therapeutic efficacy and safety of the vector is further validated in animal models.
Preclinical studies
Conduct larger and more systematic safety and toxicology studies. That is, further detailed toxicological evaluations are conducted in large animal models to confirm the long-term safety of gene therapy. To monitor the distribution, metabolism and expression of genes and vectors in vivo through pharmacokinetic and pharmacodynamic studies, and to assess efficacy.
Applications of Injectable Gene Therapy Formulation Development
- Genetic diseases. Injectable gene therapy has shown great potential for treating inherited diseases. For example, in the treatment of hemophilia, by injecting modified genes, patients can produce enough clotting factors to prevent bleeding events.
- Blood disorders. Gene injectable therapy has also made significant progress in treating blood disorders such as β-thalassemia and sickle cell anemia. By modifying the mechanism of red blood cell production, symptoms can be relieved and the quality of life of patients can be improved.
- Eye diseases. Like some congenital hereditary eye diseases, the gene injectable treatment options utilized can not only arrest the progression of the disease, but also partially restore vision in some cases.
- Cancer. In cancer treatment, gene Injectable therapies are also used to enhance the function of immune cells, making them more effective in recognizing and killing cancer cells. CAR-T cell therapy, for example, involves gene editing to transform a patient's T-cells into cells that can attack specific cancer cells and then re-inject them back into the body.
- Neurodegenerative diseases. Gene injectable therapies are also being actively researched for neurodegenerative diseases such as spinal muscular atrophy and Huntington's disease, with the aim that gene repair can slow or even reverse the progression of these diseases.
Our Platforms for Injectable Gene Therapy Formulation Development
| Platforms & Technologies |
Content Description |
| Gene delivery technology platforms |
We have established a comprehensive platform of gene delivery technologies, such as adeno-associated virus (AAV), adenovirus (AdV), lentivirus, and non-viral vectors. |
| In vivo imaging and tracking technologies |
We utilize in vivo imaging technologies to monitor the distribution and expression of gene vectors in vivo. In addition, we utilize fluorescent labeling and bioluminescence methods for tracking the localization and persistence of gene vectors and therapeutic genes. |
| Gene editing technologies |
We have established a well-established platform of gene editing technologies, including CRISPR/Cas9 for precise targeted modification of the genome, as well as TALEN and ZFN. |
Highlights of Injectable Gene Therapy Formulation Development
- We provide reliable technical support for the development of injectable gene therapy treatments, which offer new treatment options for diseases that are ineffective and difficult to treat with traditional therapies.
- We can provide customized injectable gene therapy formulation development solutions according to customer project requirements to maximize customer satisfaction.
- We can change the traits of proteins through the regulation of DNA to realize treatment from the source.
- We have rich service experience in injectable gene therapy formulation research, so clients can choose us with confidence.
Published Data
Technology: Injectable gene therapy
Journal: Proc Natl Acad Sci U S A
IF: 11.1
Published: 2022
In this study, the authors sought to determine whether high-capacity cytomegalovirus vectors (CMV) could be an effective and safe method of gene delivery of two protective factors, telomerase reverse transcriptase (TERT) and follicle suppressor (FST). The authors found that mouse cytomegalovirus (MCMV) carrying exogenous TERT or FST (MCMVTERT or MCMVFST) extended mean lifespan by 41.4% and 32.5%, respectively. The study reported that CMV was successfully used as an intranasal and injectable gene therapy system to extend life span. Specifically, this treatment significantly improved glucose tolerance and physical function and prevented weight loss and hair loss. In addition, TERT improved telomere shortening associated with aging, and both treatments prevented deterioration of the mitochondrial structure. Both intranasal and injectable formulations have performed equally well in delivering gene therapy safely and effectively to multiple organs, with long-term benefits and no carcinogenicity or adverse side effects.
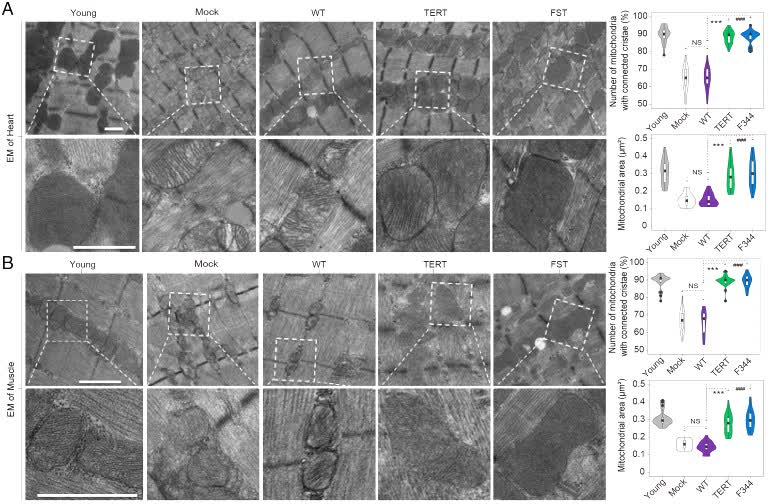 Fig.2 Preventing mitochondrial degeneration in mice. (Jaijyan DK, et al., 2022)
Fig.2 Preventing mitochondrial degeneration in mice. (Jaijyan DK, et al., 2022)
CD Formulation is a full-process, flexible formulation development, and service solution centered around the gene therapy development process, providing full support for your therapeutic development and facilitating breakthroughs at every important point. If you are interested in us, please feel free to contact us.
References
- Jaijyan DK, et al. New intranasal and injectable gene therapy for healthy life extension. Proc Natl Acad Sci U S A. 2022, 119(20):e2121499119.
Related Services


 Fig.2 Preventing mitochondrial degeneration in mice. (Jaijyan DK, et al., 2022)
Fig.2 Preventing mitochondrial degeneration in mice. (Jaijyan DK, et al., 2022) 