Inhaled Gene Therapy Formulation Development
Inquiry
Inhaled gene therapy is an innovative therapeutic approach that delivers gene drugs directly to the lungs through respiratory administration. CD Formulation has extensive research and service experience in the development of inhaled formulations for gene therapy, we have a strong understanding of the formulation process, quality control, and industrialization. We are familiar with the gene therapy formulation development process and have extensive experience in inhalation formulation development.
Advantages of Inhaled Gene Therapy Formulation
Inhaled gene therapy formulations are a method of treatment in which gene therapy drugs are delivered directly to the lungs by inhalation. This method has the following characteristics in gene therapy diseases.
- Direct drug delivery to the site of action. Inhaled gene therapy delivers the drug directly to the lungs, reducing off-target doses and systemic toxicity when treating lung diseases.
- Rapid absorption. Since the lungs have a large surface area and abundant blood flow, inhalation drug delivery can achieve rapid absorption, allowing the drug to enter the blood circulation quickly.
- Non-invasive route of administration. Inhalation administration is a non-invasive method that is safe and convenient for patients.
- Reduced side effects. By delivering the medication directly to the lungs, systemic side effects can be reduced, especially for diseases that primarily affect the lungs.
Our Services for Developing Inhaled Gene Therapy Formulation
- Target disease and gene selection
Identify the disease to be treated and the target gene. For example, targeting the CFTR gene for cystic fibrosis (CF).
- Gene vector design
Design of gene vectors suitable for inhalation drug delivery, e.g., using viral vectors or non-viral vectors (e.g., lipid nanoparticles LNP).
- In vitro experiments
Testing the efficacy and safety of gene vectors in cell culture to assess transfection efficiency and possible cytotoxicity.
- Animal model development
Development or selection of suitable animal models to mimic human diseases for initial in vivo testing.
- Preclinical research
Evaluate the safety, efficacy, dosage, and method of administration of gene therapy agents in animal models. This includes aerosolization, aerosol behavior in the respiratory tract, and post-deposition disposition processes.
- Formulation optimization
Optimization of gene therapy formulations for stability and transfection efficiency based on preclinical study results.
- Biodistribution and pharmacokinetic studies
In this segment, we study the distribution, metabolism and excretion characteristics of gene therapy formulations in vivo.
- Safety assessment
We will conduct a comprehensive toxicology study to evaluate the long-term safety and potential side effects of the gene therapy formulation.
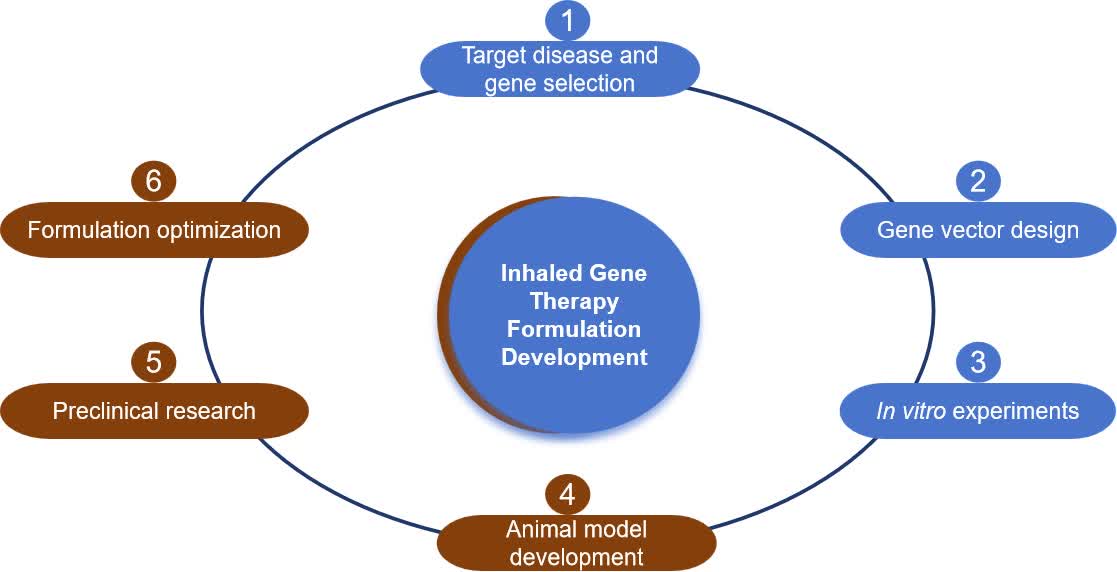 Fig.1 Inhaled gene therapy formulation development process. (CD Formulation)
Fig.1 Inhaled gene therapy formulation development process. (CD Formulation)
Applications of Inhaled Gene Therapy Formulation Development
- Rare respiratory diseases. Utilizing inhaled gene therapy has shown significant therapeutic efficacy in modifying rare respiratory diseases, and inhaled gene therapy formulations have broad applications in the treatment of respiratory diseases.
- Alpha-1 antitrypsin deficiency (AATD). Development of novel inhaled gene therapies aimed at promoting the local production of alpha-1 antitrypsin for the treatment of AATD patients.
- Congenital lung diseases. Examples include surface-active protein deficiency, cystic fibrosis, and alpha-1 antitrypsin deficiency. Inhaled mRNA delivery platforms can provide non-invasive, direct access to the lung epithelial cells and alveoli for RNA drugs, with the potential to treat these diseases.
- Pulmonary fibrosis. A novel inhaled nucleic acid nano-delivery carrier was developed to successfully deliver small interfering RNA (siRNA) to reach the lungs of fibrotic mice for the treatment of pulmonary fibrosis.
Our Technologies for Inhaled Gene Therapy Formulation Development
| Platforms & Technologies |
Content Description |
| siRNA technology |
Inhaled siRNA formulations can deliver drugs directly to the lungs, showing great potential for treating respiratory disorders. |
| Lung targeted technology |
Providing an exemplary delivery platform for the treatment of respiratory diseases, we have continually optimized our inhaled gene therapy formulations and employ its state-of-the-art vibrating mesh nebulizer technology for efficient delivery while maintaining the structural integrity. |
| Inhaled gene therapy technology platform |
We specialize in the treatment of rare respiratory diseases using inhaled gene therapy, and we have developed gene therapies that, when administered through a nebulizer, are highly effective in transducing lung epithelial cells and have long-term effects. |
Highlights of Our Inhaled Gene Therapy Formulation Development
- Continuous innovation. We are always committed to the research of new gene therapy technologies and therapies to maintain our leading position in the field of inhaled gene therapy.
- Professional knowledge and technical expertise. We have a team of experts consisting of molecular biologists, pharmacologists, bioengineers, etc., focusing on the research and development of inhaled gene therapy formulations.
- R&D capabilities. We have strong R&D capabilities to design and develop novel inhaled gene carriers and delivery systems.
- Advanced technology platform. We have developed inhaled gene therapy formulations to provide more efficient and safer gene delivery technology support to our customers.
Published Data
Technology: Non-viral vector gene delivery
Journal: Expert Opin Drug Deliv
IF: 6.6
Published: 2017
This study describes the use of non-viral vectors to formulate and deliver DNA to the lungs. It was shown that successful aerosol delivery can be achieved when the superhelical DNA structure is protected during aerosolization. In order to produce effective, cost-effective and safe formulations, a formulation strategy or compound that protects, stabilizes and efficiently transfects DNA into cells is needed. Nebulizers and dry powder inhalers are the most promising aerosol delivery methods because they involve low shear forces. In this context, it is also important to emphasize the importance of considering the "pDNA-formulation-device system" as an integral part of the development of successful nucleic acid delivery formulations.
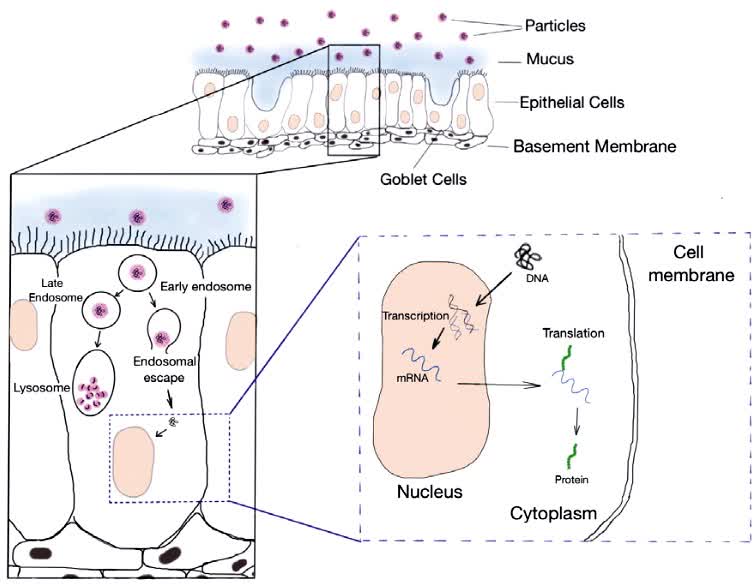 Fig.2 Delivery of DNA to lung epithelial cells. (Gomes Dos Reis L, et al., 2017)
Fig.2 Delivery of DNA to lung epithelial cells. (Gomes Dos Reis L, et al., 2017)
CD Formulation is an industry leader in gene therapy research, and we provide technical services for the development of inhaled gene therapy formulations that rely on our advanced technology platform to effectively advance gene therapy research. If you are interested in us, please feel free to contact us.
References
- Gomes Dos Reis L, et al. Inhaled gene delivery: a formulation and delivery approach. Expert Opin Drug Deliv. 2017, 14(3):319-330.
Related Services

 Fig.1 Inhaled gene therapy formulation development process. (CD Formulation)
Fig.1 Inhaled gene therapy formulation development process. (CD Formulation) Fig.2 Delivery of DNA to lung epithelial cells. (Gomes Dos Reis L, et al., 2017)
Fig.2 Delivery of DNA to lung epithelial cells. (Gomes Dos Reis L, et al., 2017)