Gene Therapy Pre-Formulation Studies
Inquiry
Pre-formulation studies play a key role in ensuring the safety, efficacy and feasibility of gene therapy formulations and are an important step in advancing gene therapy from the laboratory to clinical application. Based on our state-of-the-art technology platform and experienced team, CD Formulation aims to provide pre-formulation study services for the development of gene therapy formulations. Our goal is to study and develop optimal gene therapy pre-formulations and provide solutions to problems that may be faced during the development process.
Why Conduct Gene Therapy Pre-formulation Studies?
- Improving safety and efficacy. Pre-formulation studies can help identify and optimize the safety and efficacy of gene therapy agents to ensure that they do not cause adverse reactions in clinical applications while achieving the desired therapeutic effects.
- Vector selection and optimization. The selection of appropriate vectors is critical to the success of gene therapy. Pre-formulation studies can evaluate the properties of different vectors, such as viral and non-viral vectors, as well as their efficiency and safety in specific applications.
- Reduced immune response. Pre-formulation studies allow the design of agents that reduce the recognition and attack of therapeutic genes or vectors by the host immune system, thus improving the success of therapy.
- Improve transfection efficiency. Pre-formulation studies work to improve the transfection efficiency of gene therapy formulations, ensuring that the target cells can efficiently take up and express the therapeutic gene.
- Stability and delivery studies. Studying the physical and chemical stability of gene therapy formulations, as well as their delivery and release mechanisms in vivo, is a critical step in ensuring their stability and efficacy in clinical applications.

Explore Our Gene Therapy Pre-formulation Studies
Utilizing advanced technologies and methodologies, our team will carefully analyze and evaluate our client's project requirements, giving full consideration to a variety of factors such as stability, targeting, and safety in the development of the targeted gene therapy formulations. We will go through a series of experiments and tests to ultimately provide customers with satisfactory gene therapy formulation products. Throughout the pre-formulation study process, our research team continuously optimizes the analytical methods, aiming to provide high-quality technical support for subsequent formulation development. Our pre-formulation research services include but are not limited to the following.
Vector selection and development
Vectors for gene therapy are key tools for delivering therapeutic genes to target cells. Our research may focus on the selection and modification of different types of viral vectors (e.g., retroviruses, adeno-associated viruses, lentiviruses, etc.) and non-viral vectors (e.g., liposomes, nanoparticles, etc.) to improve their delivery efficiency and to reduce immunogenicity.
Optimization of delivery systems
Different delivery systems have a significant impact on the efficacy of gene therapy. Key points of our research include but are not limited to transfection efficiency, cytotoxicity, tissue specificity, etc.
Delivery pathway studies
How the gene enters the target cells or tissues is a key issue in the pre-formulation study, and we need to choose the appropriate delivery route according to the target and drug properties, such as systemic delivery, gene gun, etc.
Design and optimization of therapeutic genes
This process involves selecting or designing gene sequences capable of treating a specific disease, e.g., for hemophilia, clotting factor genes may be designed to compensate for defective genes. In addition, we optimize gene sequences, such as the selection of promoters and the use of enhancers.
Safety evaluation
In the pre-formulation stage, we must evaluate the potential risks of gene therapy, including insertion mutations, immune response non-target effects, etc. We focus on confirming whether there will be any adverse or immune reactions, including acute toxicity and chronic toxicity studies, immunogenicity assessment, etc.
Efficacy studies
To study the efficacy of gene therapy, including tests in vitro cell cultures and animal models to determine the expression levels of therapeutic genes and therapeutic effects.
Stability studies
Ensure that gene vectors and gene therapy formulations remain stable during storage and transportation, including but not limited to solution stability, Lyophilization Stability, and storage condition studies.
Our Technologies for Gene Therapy Pre-Formulation Studies
| Technologies |
Content Description |
| Biochemical analysis technology platform |
We have established a well-established technical platform for biochemical analysis such as high-performance liquid chromatography (HPLC), mass spectrometry (MS), gel electrophoresis, etc. to support pre-formulation studies. |
| Cell culture technology platform |
We culture primary cells and cell lines to study the infection efficiency and transgene expression of vectors on different cell types. In addition, we perform cell activity analysis by detecting the cytotoxicity of vectors. |
| Gene editing technology platform |
Includes the use of gene editing technologies such as CRISPR/Cas9 to make precise genetic modifications for the treatment of inherited diseases or cancers. |
Highlights of Our Gene Therapy Pre-Formulation Studies
- Our state-of-the-art equipment and technology provide efficient and reliable research services for gene therapy pre-formulation, which can effectively reduce costs and increase productivity.
- We offer a range of customized testing options to meet the pre-development needs of gene therapy formulations, including a variety of gene delivery vectors, delivery modes, formulation screening tests, etc. We have a team of researchers with expertise in molecular biology, pharmaceuticals, and proteins.
- Has a research team with extensive expertise in various fields such as molecular biology, pharmaceuticals and proteins, and many years of research experience in pre-formulation studies in gene therapy.
- We provide flexible design solutions according to customer requirements to ensure that we meet their project requirements.
Published Data
Technology: A study of rAAV-mediated gene therapy pre-formulation
Journal: J Pharm Sci
IF: 3.7
Published: 2021
In this study, the authors investigated the effect of freezing and thawing speed on the recombinant adeno-associated virus (rAAV2) as a model serotype. Slow thawing rates affected rAAV2 colloidal stability in phosphate-based buffer systems. The pre-formulation workflow indicated that rAAV2 has the greatest tendency to aggregate between pH 5.5 and 6.5. Thus, the overlap between the pH range of maximum aggregation propensity and the acidic pH change in phosphate-based buffer systems during freezing and thawing appears to be responsible for the 42-75% decrease in rAAV2 concentration. This effect appeared to be fully mitigated by replacing the phosphate buffer system with an alternative buffer system such as Tris. The results reported in this study emphasize the associated risks and provide preliminary guidance for dealing with early frozen rAAV-mediated gene therapy.
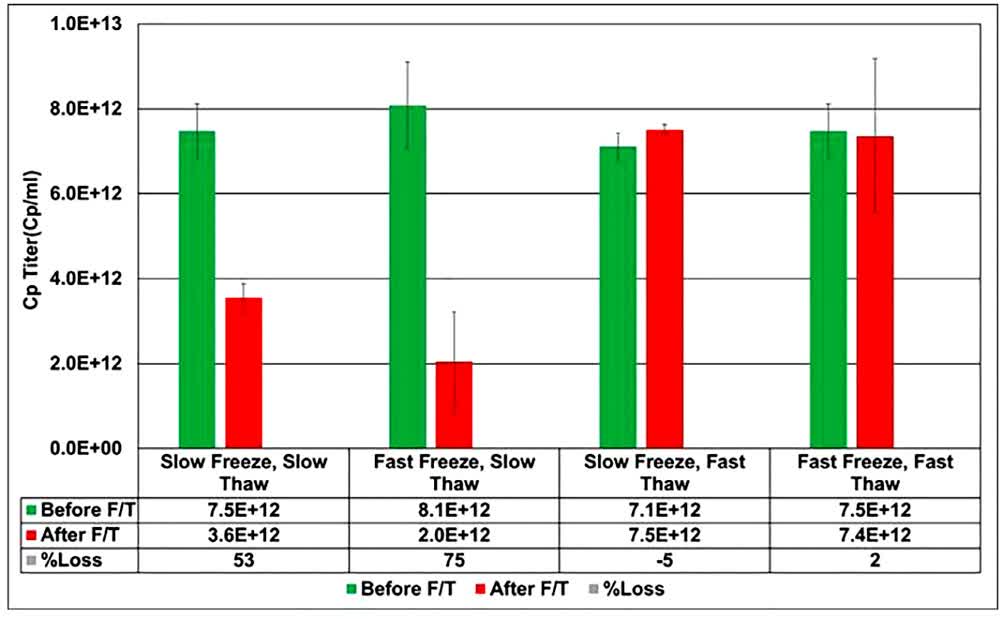 Fig.2 Effect of freezing and thawing rates on the recombinant adeno-associated virus. (Pandharipande P, et al., 2021)
Fig.2 Effect of freezing and thawing rates on the recombinant adeno-associated virus. (Pandharipande P, et al., 2021)
CD Formulation provides reliable technical support and solutions for pre-formulation studies in gene therapy, greatly facilitating the development of gene therapy formulations. If you are interested in us, please feel free to contact us.
References
- Pandharipande P, et al. Considerations for Buffering Agent Selection for Frozen rAAV2 Mediated Gene Therapy Products. J Pharm Sci. 2021, 110(10):3535-3539.
Related Services


 Fig.2 Effect of freezing and thawing rates on the recombinant adeno-associated virus. (Pandharipande P, et al., 2021)
Fig.2 Effect of freezing and thawing rates on the recombinant adeno-associated virus. (Pandharipande P, et al., 2021)